2-Dimethylaminoisopropyl chloride hydrochloride - Reaction / Application on synthetic works
Hydrochloride salt of 2-dimethylaminoisopropyl chloride was used in the synthesis of analogues of lipophilic chalcones, which act as antitubercular agents.
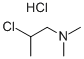
The following example is about its application on the synthesis of substituted benzo[h]quinazolines as anti-tubercular agents [1]
In a 100mL R.B. Flask, 4-(2-amino-5,6-dihydro-8-methoxybenzo[h]quinazolin -4-yl)phenol (4, 0.2g, 0.6mmol) was dissolved in the mixture of acetone (40mL) and chloroform (25mL) at 80°C. After stirring for ten minutes, anhydrous K2CO3 (0.4g) and substituted amine ethyl chloride hydrochloride 5 (0.13g, 1.07mmol) was added in reaction mixture. The progress of reaction was monitored by thin layer chromatography. After the completion of reaction, solvent was evaporated and residue was diluted with chloroform and extracted with water. The organic phase was filtered, evaporated and dried. The crude was purified by silica gel column chromatography with a mixture of chloroform–hexane (10percent) to give the desired product.
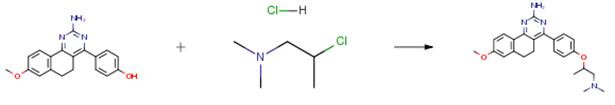
The following example is about its application on the synthesis of synthesis of 3,5-dihydroxy-7,8-dimethoxy-2-(4- methoxyphenyl)benzopyran-4-one derivatives as anticancer agents [2]
In a round bottom flask, the starting material (0.1 g, 0.29 mmol) was dissolved in dry acetone (20 ml). To this mixture,2-N-piperidinoethylchloride hydrochloride (0.06 mL, 0.33 mmol) anhydrous K2CO3(0.23 g, 1.60 mmol) was added. The reaction was allowed to reflux for 5-6 h. After completion of the reaction, reaction mixture was filtered and filtrate was evaporated. The obtained residue was extracted with ethyl acetate and water. Organic layer was dried on anhydrous sodium sulphate and concentrated to oily crude material. The crude product was purified by column chromatography over basic alumina using ethyl chloroform: methanol as eluent.
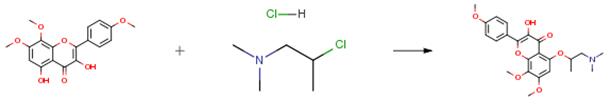
The following example is about its application on the synthesis of triazolone derivatives [3]
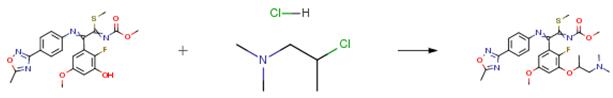
After adding 217 mg of potassium carbonate and 124 mg of (2-chloropropyl)dimethyl amine hydrochloride to a 3 ml DMF solution containing 300 mg of [2-(2-fluoro-3- hydroxy-5-methoxyphenyl)-2-[4-(5-methyl-[1,2,4]oxadiazol-3-yl)phenylimino]-1-methylsulfanylethylidene]carbamic acid methyl ester, the mixture was stirred at an external temperature of 80°C for 20 hours and 30 minutes. Next, 20 mg of tetrabutylammonium iodide was added to the reaction mixture and stirring was continued at the same temperature for 5 hours and 30 minutes. The reaction mixture was concentrated, and the obtained residue was crudely purified by NAM silica gel column chromatography (chloroform-methanol) to give 210 mg of a crude product. After dissolving 50 mg of the 210 mg of obtained crude product in 2 ml of DMF, 15 mg of 2-hydrazinopyridine and 0.025 ml of triethylamine were added to the solution and the mixture was stirred at 80° C for 10 hours under a nitrogen atmosphere. The reaction mixture was then concentrated. Next, 1 ml of methanol and 0.014 ml of acetic acid were added to dissolve the obtained residue. To this solution there was added 30 mg of sodium cyanotrihydroborate, and the mixture was stirred at room temperature for 12 hours. The reaction mixture was then concentrated. Next, 1.5 ml of a methanol:water:acetic acid=1:1:1 mixed solvent was added to dissolve the obtained residue. After adding 50 mg of iron powder to the solution, the mixture was stirred at 60° C. for 14 hours and 45 minutes under a nitrogen atmosphere. After filtering the reaction mixture, it was purified by reverse-phase high performance liquid chromatography (acetonitrile-water, 0.1percent trifluoroacetic acid), to give the product (3.98 mg) as a light brown oil.
The following example is about its application on the synthesis of substituted benzodiazepin-10-ones [4]
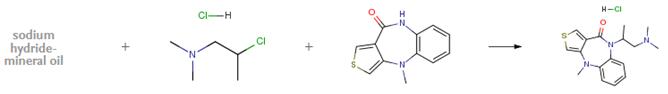
A mixture of 0.47 g. of 55 percent sodium hydride-mineral oil dispersion and 0.83 g. of 2-dimethylamino-1-methylethylchloride hydrochloride in 30 ml. of dry dimethyl formamide is stirred at room temperature for 0.5 hours. To the mixture is added 0.6 g. of 4,9-dihydro-4-methyl-10H-thieno[3,4-b] [1,5]benzodiazepin-10-one and stirring is continued for 18 hours. The reaction mixture is cooled, quenched with water and extracted with chloroform. The dried chloroform extracts are concentrated to an oil which is chromatographed on silica gel preparative thin layer chromatographic (TLC) plates using 10:1 benzene:methanol to give a yellow oil. The oil is dissolved in ethanol and treated with anhydrous hydrogen chloride followed by dilution with ether to give a solid, which is recrystallized from ethanol to give white crystals, m.p. 280°-282°C.
References
1. Maurya HK, Verma R, Alam S, Pandey S, Pathak V, Sharma S, Srivastava KK, Negi AS, Gupta A. Studies on substituted benzo[h]quinazolines, benzo[g]indazoles, pyrazoles, 2,6-diarylpyridines as anti-tubercular agents. Bioorganic and Medicinal Chemistry Letters, 2013, 23(21):5844-5849.
2. Singh S, Ahmad A, Raghuvanshi DS, Hasanain M, Agarwal K, Dubey V, Fatima K, Tandon S, Gupta A. Synthesis of 3,5-dihydroxy-7,8-dimethoxy-2-(4- methoxyphenyl)benzopyran-4-one derivatives as anticancer agents. Bioorganic and Medicinal Chemistry Letters, 2016, 26(21):5322-5327.
3. Eisai R&D Management Co., Ltd. Triazolone derivatives. US2008/15199[P], 2008, A1,Page column 50.
4. American Cyanamid Company. Substituted benzodiazepin-10-ones and method of use. US3953430[P], 1976, A.
Related articles And Qustion
Lastest Price from 2-Dimethylaminoisopropyl chloride hydrochloride manufacturers

US $3.00-0.10/kg2025-10-31
- CAS:
- 4584-49-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $1.00/g2025-04-21
- CAS:
- 4584-49-0
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 100kg