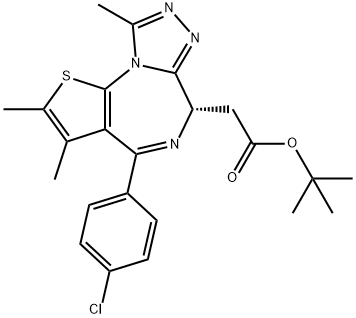
(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-t - Reaction / Application on synthetic works
JQ1 is a thienotriazolodiazepine and a potent inhibitor of the BET (bromodomain and extra-terminal motif) family of bromodomain proteins which include BRD2, BRD3, BRD4, and the testis-specific protein BRDT in mammals[1]. BET inhibitors structurally similar to JQ1 are being tested in clinical trials for a variety of cancers including NUT midline carcinoma. [2] It was developed by the James Bradner laboratory at Brigham and Women's Hospital and named after chemist Jun Qi. The chemical structure was inspired by patent of similar BET inhibitors by Mitsubishi Tanabe Pharma. Structurally it is related to benzodiazepines. While widely used in laboratory applications, JQ1 is not itself being used in human clinical trials because it has a short half life. It is an important organic intermediate to synthetize substituted thienotriazolodiazepine products.
The following example is about its application on the synthesis of heterobifunctional compounds with improved specificity for the bromodomain of BRD4 [2]
The JQ1 (1 g, 2.2 mmol) was dissolved in 40% TFA/DCM solution(24 mL) and stirred at room temperature for 5 h. The reaction mixture was concentrated and dissolved in EtOAc. The organic layer was washed with water and brine, dried over MgSO4, and concentrated. The residue was purified by column chromatography to give the product (900 mg, quant.) as a yellow solid.
The following example is about its application on the synthesis of 6H-Thieno[3,2-f][1,2,4] triazolo[4,3-a][1,4]diazepines [3]

JQ1 (4.57 g, 10 mmol) was dissolved in MeOH (0.25 M). conc.H2SO4(50 drops) was added to the solution. The mixture was refluxed overnight. The mixture was concentrated in vacuo, poured into water, extracted with AcOEt, and washed with brine. The organic layer was dried over Na2S04, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography (AcOEt/MeOH) to give the product 3.93 g (95 %).
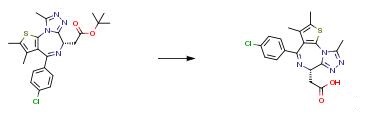
The following example is about its application on the synthesis of 2-thiazolidinones as inhibitors of the histone reader BRD4 bromodomain [4]

A solution of 1.6 g (3.5 mmol) of tert-butyl[(S)-4-(4-chlorophenyl)-2,3,9- trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl]acetate in 25 ml HCl in dioxane (4N) was stirred overnight at RT. The solvent was removed completely under vacuum and the product was obtained as a solid. 1.53 g.
References
1. Kim SA, Go A, Jo SH, Park SJ, Jeon YU, Kim JE, Lee HK, Kim JH, Hwang JY. A novel cereblon modulator for targeted protein degradation[J]. European Journal of Medicinal Chemistry, 2019, 166:65-74.
2. Dana-Farber Cancer Institute, Inc. Nowak RP. Fischer ES. Gray NS. Zhang T, He Z. Heterobifunctional compounds with improved specificity for the bromodomain of BRD4. WO2019/79701[P], 2019, A1, Paragraph 0351-0353.
3. Bayer Intellectual Property Gmbh. Schmees N, Kuhnke J, Haendler B, Lienau P, Fernandez-Montalvan AE, Lejeune P, Siegel S, Scott W. 6H-Thieno[3,2-f][1,2,4] triazolo[4,3-a][1,4]diazepines. US2014/213575[P], 2014, A1, Paragraph 0449, 0450.
4. Zhao L, Cao D, Chen T, Wang Y, Miao Z, Xu Y, Chen W, He J, Shen J. Fragment-based drug discovery of 2-thiazolidinones as inhibitors of the histone reader BRD4 bromodomain[J]. Journal of Medicinal Chemistry, 2013, 56(10): 3833-3851.
You may like
Lastest Price from (+)-JQ-1 manufacturers
![12688524-70-4 (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate](https://img.chemicalbook.com/ProductImageEN/2020-3/Small/cfa6bbcf-975a-4c2c-9364-ceeaab9583cd.jpg)
US $1.00/KG2020-03-06
- CAS:
- 12688524-70-4
- Min. Order:
- 1KG
- Purity:
- 97%-99.9%
- Supply Ability:
- 200kg
![1268524-70-4 (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate](https://img.chemicalbook.com/ProductImageEN/2020-1/Small/6dce4ff0-6df7-49e7-aaa6-e88d8213cc93.jpg)
US $7.00/KG2020-01-14
- CAS:
- 1268524-70-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100KG