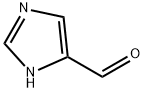
1H-Imidazole-4-carbaldehyde - Reaction / Application on synthetic works
4-Imidazolecarboxaldehyde (1H-Imidazole-4-carbaldehyde, 4(5)formylimidazole, 4-formylimidazole) is a 4-formyl derivative of imidazole used in the preparation of C17,20-lyase inhibitor for the treatment of androgen-dependent prostate cancer. 4-Imidazolecarboxaldehyde is also used in the synthesis of other biologically active compounds such as antimalarial drugs.
The following example is about its application on the synthesis of a imidazole derivative[1]
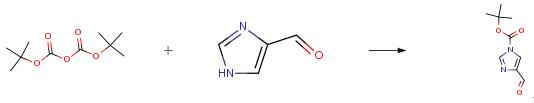
To a solution of 4-formylimidazole (10.0 g, 104.1 mmol) in tetrahydrofuran (100 mL) were added triethylamine (12.6 g, 124.9 mmol) and a catalytic amount of 4-dimethylaminopyridine (2.5 g, 20.8 mmol). A solution of di-t-butyl-dicarbonate (27.3 g, 124.9 mmol) in THF (50 mL) was added dropwise thereto at 30°C or below, and the mixture was stirred at room temperature for 1 hr or more. After the reaction, water (100 mL) was added 35 dropwise thereto at 30°C or below to quench the reaction, and the ethyl acetate (200 mL) was added thereto. The organic layer was separated, and concentrated under reduced pressure to the volume of about 30 mL. To the residue was added diisopropyl ether (100 mL), and the mixture was concentrated to the volume of about 20 mL under reduced pressure. These operations were repeated twice to adjust the volume to about 20 mL. The crystals were collected by filtration,., and washed with diisopropyl ether (20 mL) , and then washed twice with water (50 mL) . The obtained wet crystals were dried under reduced pressure at an outside temperature of 50°C to give t- butyl 4-formyl-lH-imidazole-l-carboxylate (16.0 g, 81.5 mmol). yield 78 percent.
The following example is about its application on the synthesis of a tetrahydroimdidazopyrazine derivative as CGRP receptor antagonists [2]
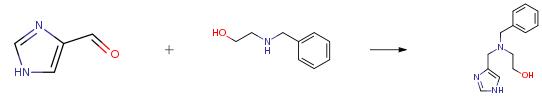
A flask was charged with lH-imidazole-4-carbaldehyde (5.0 g, 52 mmol), tetrahydrofuran (75 mL), and 2- (benzylamino)ethanol (9.44 g, 62.4 mmol). The reaction was stirred at room temperature for 1 h. To this was added sodium triacetoxyborohydride (13.2 g, 62.4 mmol) in one portion. A significant exotherm was noted. After 10 min, the solution had become a thick suspension which required occasional agitation by hand. The reaction was stirred at room temperature overnight. The reaction was concentrated, dissolved in water (100 mL), made basic with an aqueous solution of potassium carbonate, and extracted into dichloromethane (3X). The organics were dried over potassium carbonate and concentrated. Column chromatography (5percent MeOH/DCM 10percent MeOH/DCM -> 90: 10: 1 DCM/MeOH/2M ammonia in methanol) gave 10.2 g (85 percent) as a viscous oil.
The following example is about its application on the synthesis of a mutant IDH inhibitor [3]
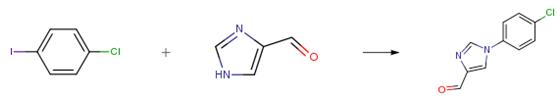
1H-imidazole-4-formaldehyde (5.0 g, 52.03 mmol, 1.0 eq), 1-chloro-4-iodobenzene (18.6 g, 78.05 mmol, 1.5 eq), cesium carbonate (34.0 g, 104.06 mmol, 2.0 eq), cuprous iodide (500 mg, 2.60 mmol, 0.05 eq), trans-(1R,2R)-N,N’dimethyl 1,2-cyclohexane diamine (1.5 g, 10.04 mmol, 0.2 q) and N,N-dimethylformamide (100 mL)were sequentially added to a dry 250 mL three-neck flask at room temperature, the air in the system were replaced with nitrogen for three times, and stirred at 110°C for 18 hours under nitrogen. After the reaction was completed, the reaction mixture was cooled to 0°C, a saturated aqueous solution of ammonium chloride (500 mL) was added, and ethyl acetate (1200 mL) was used for extraction, the organic phase was dried over sodium sulfate and concentrated under reduced pressure to give a crude product. The crude product was purified through a silica gel column (petroleum ether: ethyl acetate = 2:1) to give product 1-(4-chlorophenyl)- 1H-imidazole-4-formaldehyde (6.0 g, yellow solid), yield 56 percent.
The following example is about its application on the synthesis of a imidazole derivative [4]
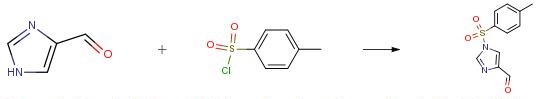
4-Formylimidazole (20.0 g, 208.14 mmol) and p- toluene sulfonyl chloride (43.7 g, 229.0 mmol) were suspended in N, N-dimethylacetamide (2.00 mL). To the obtained suspension was added dropwise triethylamine (23.2 g, 229.0 mmol) at 10°C or below, and the mixture was stirred at 10°C or below for 1 hr or more. To the reaction mixture was added n-heptane (60 mL) at 30°C or below. To the obtained solution was added dropwise water (240 mL) at 30°C or below for crystallization. The mixture was stirred at room temperature for 1 hr or more to give crystals. The obtained crystals were collected by filtration, and washed with water (300 mL) to give wet crystals. The obtained wet crystals were dried under reduced pressure at an outside temperature of 50°C to give l-tosyl-4- formyl-lH-imidazole (44.2 g, 176.6 mmol). yield 85 percent.
References
1. Takeda Pharmaceutical Company Limited. Kawabata Y, Sawai Y, Kanno K, Sawada N. Production method of imidazole derivatives. WO2012/173280[P], 2012, A1, Page column 30-31.
2. Bristol-Myers Squibb Company. Degnan AP. Tetrahydroimdidazopyrazine derivatives as CGRP receptor antagonists. WO2014/62548[P], 2014, A1, Page column 23.
3.Shanghai Haihe Pharmaceutical Co. Ltd. Shanghai Institute of Materia Medica, Chinese Academy of Sciences. Jiang L, Geng M, Zheng Q, Huang M, Wan H, Liu F, Ding J. Compound having mutant IDH inhibitory activity, preparation method and use thereof. EP3434674[P], 2019, A1, Paragraph 0145, 0146.
4. Takeda Pharmaceutical Company Limited. Kawabata Y, Sawai Y, Kanno K, Sawada N. Production method of imidazole derivatives. WO2012/173280[P], 2012, A1, Page column 29-30.
You may like
Related articles And Qustion
Lastest Price from 1H-Imidazole-4-carbaldehyde manufacturers

US $0.00-0.00/kg2025-04-24
- CAS:
- 3034-50-2
- Min. Order:
- 1kg
- Purity:
- 99.32%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 3034-50-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons