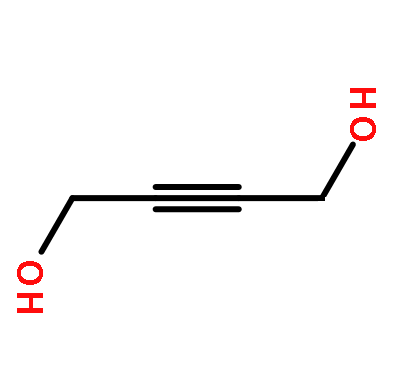
2-Butyne-1,4-diol:Uses,Synthesis and Hazard
2-Butyne-1,4-diol is a white solid in various forms. It decomposes on heating. It is toxic and corrosive fumes. Reacts violently with anhydrides, acid chlorides, mercury salts and alkaline hydroxides or chlorides. This generates explosion hazard.

Uses
2-Butyne-1,4-diol is an important specialty chemical intermediate; it is used in the manufacture of endosulfan, vitamins A and B6. It also has wide-ranging applications in the paper and textile industries and in the resin manufacture.[1]
Synthesis
1,4-Butynediol can be produced in the Reppe synthesis, where formaldehyde and acetylene are the reactants:
2 CH2O + HC≡CH → HOCH2CCCH2OH
Several patented production methods use copper bismuth catalysts coated on an inert material. The normal temperature range for the reaction is 90 °C up to 150 °C, depending on the pressure used for the reaction which can range from 1 to 20 bar.
Chemical study
Commercial grade 2-butyne-1,4-diol has been used in electroplating for several years. In laboratory experiments, its presence in the electrolyte increases the current efficiency of zinc electro-winning. Its chemical behaviour in solution is not well known. The present paper indicates that the brownish technical grade 2-butyne-1,4-diol contains the monomer, the dimer and some trimer. Pure monomeric 2-butyne-1,4-diol is a white solid obtained by evaporation of the technical grade product. The monomer is slowly transformed into dimer and possibly into a trimer when dissolved in water. Various analytical techniques were used in the study of this system. Factor analysis with column cross-validation was applied to chromatographic data to help in the resolution of the system.
Hazard
2-butyne-1,4-diol is Corrosive. Inhalation may cause lung oedema, but only after initial corrosive effects on eyes and/or airways have become manifest. Medical observation is indicated. may have effects on the blood. This may result in anaemia. It may have effects on the kidneys and liver. This may result in tissue lesions. Repeated or prolonged contact may cause skin sensitization.
Reference
[1] M.M. Telkar.“Selective hydrogenation of 2-butyne-1,4-diol to 2-butene-1,4-diol: roles of ammonia, catalyst pretreatment and kinetic studies.”Applied Catalysis A: General 216 1 (2001): Pages 13-22.
You may like
Related articles And Qustion
See also
Lastest Price from 2-Butyne-1,4-diol manufacturers

US $0.00/kg2025-08-22
- CAS:
- 110-65-6
- Min. Order:
- 1000kg
- Purity:
- 99%
- Supply Ability:
- 50 ton per month

US $10.00-8.00/KG2025-04-21
- CAS:
- 110-65-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt