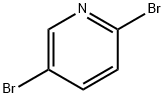
2,5-Dibromopyridine: Applications in Organic Chemistry and Green Synthesis
Description
Pyridine and its derivatives is distributed in nature widely.Many plant constituents are as all contained pyridine ring compound in the structure of alkaloid etc., they are the bases producing many important compound, are indispensable raw materials during medicine, agricultural chemicals, dyestuff, tensio-active agent, rubber ingredients, fodder additives, foodstuff additive, tackiness agent etc. are produced. 2,5-Dibromopyridine is the important intermediate of organic synthesis, is mainly used in medicine intermediate, organic synthesis, organic solvent, also can be applicable to the aspects such as dye production, pesticide producing and spices.

synthetic method of 2,5-Dibromopyridine
By 18.82g(0.2mol) PA and 30.63g(0.3mol) aceticanhydride joins 200ml tetra-mouthfuls of oil baths and is warming up to backflow, reaction is followed the tracks of to reacting completely with thin-layer chromatography, when question response liquid temp is down to 20-25 DEG C, slow dropping 35.2g(0.22mol) bromine, drip and finish, in 50 DEG C of reactions 2.5 hours.Add water to after solid dissolves completely in system, slowly drip the sodium hydroxide solution of 80ml40%, have a large amount of solid to generate, continue reaction 30 minutes, suction filtration, drying, obtains 2-amino-5-bromopyridine 14.1g with ethyl alcohol recrystallization, yield 65%(molar yield).
The hydrobromic acid solution of 50ml48% is added in the 200ml there-necked flask that agitator, thermometer are housed, by 6.9g(0.048mol) cuprous bromide is dissolved in hydrobromic acid solution, ice-water bath keeps temperature 0 DEG C slowly to add 6g(0.04mol) 2-amino-5-bromopyridine, keep temperature 20 minutes, the saturated sodium nitrite solution 10ml of slow dropping, adds rear stirring 3.5 hours, by 40% sodium hydroxide solution neutralization reaction to pH=7-8, underpressure distillation obtains 2,5-Dibromopyridine, and yield is 64%.
References:
[1] R. J. CHAMBERS A M. Regiospecific Carboalkoxylation of 2,5-Dibromopyridine[J]. Synthetic Communications, 1997, 27 1: 515-520. DOI:10.1080/00397919708006053.Lastest Price from 2,5-Dibromopyridine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 624-28-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 624-28-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT