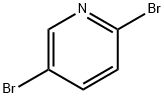
Description
Pyridine and its derivatives are distributed widely by nature. Many plant constituents are as contain pyridine ring compounds in the structure of alkaloids, etc.; they are the bases producing many vital compounds and are indispensable raw materials during medicine, agricultural chemicals, dyestuff, tension-active agents, rubber ingredients, fodder additives, foodstuff additives, tackiness agents, etc. are produced.2,5-dibromo pyridine is an important intermediate of organic synthesis and is mainly used in medicine intermediate, organic synthesis, and organic solvent, can also be applicable to aspects such as DYE PRODUCTION, pesticide production and spices. Selective monolithiation of 2,5-dibromopyridine at either the 2-position or the 5-position is reported. Solvent and concentration strongly influence the selectivity. Coordinating solvents and higher concentration favour the 5-position, while non-coordinating solvents and lower concentration favour the 2-position[1].
Preparation
To synthesize 2,5-dibromopyridine, follow these steps:
Add 2-amino-5-bromopyridine (13.0 kg) into a water-cooled solution at 10 °C.
Slowly add 47% aqueous hydrogen bromide (37 L) to the mixture.
Introduce liquid bromine (11 L) into the mixture.
Maintain the reaction temperature below 10 °C.
Prepare a solution of NaNO2 (16.1 kg) and H2O (19 L) and add it dropwise to the mixture while keeping the temperature between 0-5 °C.
Stir the reaction mixture for 30 minutes.
Treat the mixture with a solution of NaOH (28.0 kg) in water (30 L) at a controlled rate below 20-25 °C.
Extract the reaction mixture with diethyl ether (3 × 40 L).
Dry the organic layer with anhydrous Na2SO4.
Remove the desiccant by filtration.
Evaporate the resulting filtrate under reduced pressure to dryness.
Suspend the residue in heptane (10 L).
Collect the target product molecule, 2,5-dibromopyridine, by filtration.
Synthesis method of 2,5-dibromopyridine