Sodium Cyclamate: A Deep Dive into Its Uses and Safety in Modern Chemistry
Introduce
Sodium Cycloamate, commonly known as cyclohexyl sweetener, is an artificially synthesized nonnutritive sweetener. In 1937, it was first discovered by American chemist Michael Sveda. Due to its high sweetness and low cost, Sodium Cyclamate has quickly been widely used in various fields such as food, beverage, and medicine. Although it is popular in the sweetener market, its safety issues are also of great concern, and laws and regulations in different countries have different restrictions on its use. Next, we will introduce some information about Sodium Cyclamate and its precautions to help chemical workers better use it.

Figure 1 Characteristics of Sodium Cyclamate
Nature
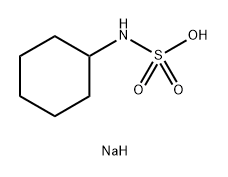
The chemical formula of Sodium Cyclamate is C ₆ H ₁ ₂ NNaO ∝ S, with a molecular weight of approximately 201.22 g/mol. It appears as white crystals or crystalline powder, with no or slightly bitter taste, and is highly soluble in water, with a solubility of approximately 1 g/5 mL of water (25 ℃). Under dry conditions, Sodium Cycloamate exhibits stable properties and is not easily decomposed; But it may hydrolyze into cyclohexylamine and sulfamino in acidic environments. Its sweetness is about 30-50 times that of sucrose, and it is commonly used in low-calorie drinks, candies, and medicines. Compared to other sweeteners, Sodium Cycloamate has a mild taste and does not mask the natural flavor of other ingredients.
Main components
The main component of Sodium Cycloamate is sodium cyclohexylamine sulfonate, which is an artificially synthesized compound made by introducing sulfonic acid groups onto cyclohexylamine. Usually, Sodium Cycloamate is not used alone as a sweetener but is combined with other sweeteners such as saccharin or aspartame to achieve a more ideal taste and sweetness. This combination not only reduces costs but also minimizes the taste defects of a single sweetener.
Purpose
Sodium Cycling is widely used in the food and beverage industry due to its high sweetness and low cost, especially for the production of low-calorie and sugar-free products such as sugar-free soft drinks, candies, ice cream, and jam. In addition, in the pharmaceutical field, Sodium Cycloamate is used in oral medications such as pills and syrups to help mask the bitterness of the medication and enhance the user experience. For people with diabetes and those who control their weight, Sodium Cyclomate is an ideal sweet substitute.
Storage method
Sodium cyclamate should be stored in a sealed, dry, and cool environment to prevent moisture absorption and clumping. Although it is relatively stable in a dry state, it may decompose under high temperature and high humidity conditions, affecting the sweetness effect. Therefore, industrial storage typically requires avoiding acidic or strong oxidizing agents to prevent chemical reactions. In addition, Sodium Cycloamate powder is prone to scattering, and it is recommended to wear a protective mask during handling to avoid inhalation.
Security controversy
Sodium Cyclamate is widely favored for its affordability and high sweetness, but its safety has been a concern since the 1960s. Research suggests that Sodium Cycloamate may decompose into cyclohexylamine in the body, which is believed to be carcinogenic. Based on this, the US Food and Drug Administration (FDA) banned its use in 1969. However, Europe and China have re-evaluated and allowed the use of Sodium Cycloamate within strict dosage limits.
Conclusion
As a low calorie sweetener, Sodium Cycloamate has significant advantages in the fields of food, beverage, and medicine. However, given its safety controversies, researchers and regulatory agencies still need to conduct in-depth research on its impact on health and reasonably regulate its use. The risk level of Sodium Cycloamate varies in different regions, application scenarios, and dosages. For chemical workers and professionals in related industries, a thorough understanding of their properties, ingredients, and usage methods, as well as strict compliance with regulatory requirements, is the key to ensuring product safety.
References:
[1] ZHENHUA CHEN . Toxicity of food sweetener-sodium cyclamate on osteoblasts cells[J]. Biochemical and biophysical research communications, 2019, 508 2: 339-666. DOI:10.1016/j.bbrc.2018.11.172.[2] P.G BRANTOM P G I F Gaunt. Long-term toxicity of sodium cyclamate in mice[J]. Food and cosmetics toxicology, 1973, 11 5: 713-934. DOI:10.1016/0015-6264(73)90132-6.
Lastest Price from Sodium N-cyclohexylsulfamate manufacturers

US $0.00-0.00/kgs2025-06-28
- CAS:
- 139-05-9
- Min. Order:
- 25kgs
- Purity:
- ≥99.0%
- Supply Ability:
- 100 tons

US $10.00/kg2025-04-21
- CAS:
- 139-05-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100ton