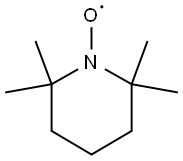
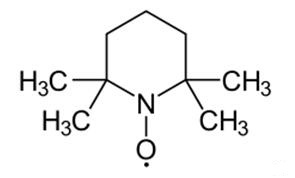
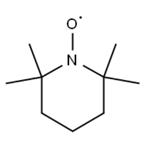
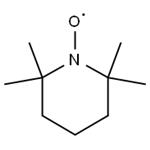
2,2,6,6-Tetramethylpiperidinooxy:a radical initiator
Introduction
2,2,6,6-Tetramethylpiperidinooxy is a chemical compound used as a radical initiator in various chemical reactions, particularly in organic synthesis. It is known for its ability to catalyze the oxidation of primary alcohols to aldehydes. 2,2,6,6-Tetramethylpiperidinooxy is widely employed in research and industrial processes due to its stability and selectivity in free radical reactions. Additionally, its derivatives have applications in polymer chemistry and as stabilizers in certain chemical reactions. Careful handling and storage are essential due to its sensitivity to air and moisture, which can affect its reactivity. 2,2,6,6-Tetramethylpiperidinooxy is generally considered safe when handled and used with proper precautions. However, like many chemical compounds, it poses potential risks, and adherence to safety guidelines is essential.
Application
2,2,6,6-Tetramethylpiperidinooxy serves diverse roles in applications ranging from organic synthesis to materials science. As a radical initiator and oxidation catalyst, 2,2,6,6-Tetramethylpiperidinooxy facilitates the controlled oxidation of alcohols, pivotal in organic synthesis and fine chemical production. Its impact extends to polymer chemistry, where it enables controlled radical polymerization. In the paper industry, 2,2,6,6-Tetramethylpiperidinooxy, in combination with sodium hypochlorite, acts as a pulp bleaching agent. Widely utilized in biomedical research, 2,2,6,6-Tetramethylpiperidinooxy and its derivatives serve as stable nitroxide radicals for electron paramagnetic resonance spectroscopy. In polymer and plastic production, 2,2,6,6-Tetramethylpiperidinooxy derivatives function as antioxidant stabilizers. Additionally, 2,2,6,6-Tetramethylpiperidinooxy finds use in electrochemical reactions, surface modification, dyes, and pigments. Its versatile applications highlight its significance in various industries, necessitating careful handling and adherence to safety guidelines.
1. Human serum albumin has been treated with the spin-labeling reagent indicated in the title. Ultraviolet spectral studies of the protein so modified suggest that reaction takes place at lysine and tyrosine sidechains; kinetic experiments indicate that there are two especially reactive amino groups of the protein which are preferentially modified. Evidence is presented that these groups include the one acetylated by aspirin (Lys-199) or those arylated by 2.6-dinitro-4-trifluoromethylbenzenesulfonate. Esr experiments show that bound spin labels have about the same correlation time expected for overall tumbling of the protein; ESR observations indicate that molecular freedom near the spin labels is not increased when the protein is transferred to 8 M urea1.
2. A poly (ethylene sulfide) backbone is introduced as the main chain of a radical polymer. Anionic ring-opening polymerization of an episulfide monomer substituted with 2,2,6,6-Tetramethylpiperidinooxy, a robust nitroxide radical, yields the corresponding polythioether. Compared to the traditional poly (ethylene oxide) backbone, the new polymer shows a lower glass transition temperature (−10 °C), and about threefold higher solid-state ionic conductivity. The polythioether is also shown to improve the charge/discharge properties of a cathode in solid-state lithium-ion batteries2.
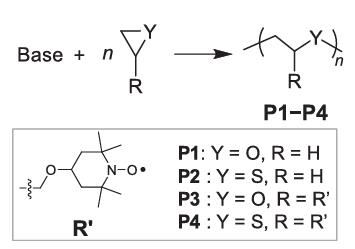
Figure 1. Syntheses of poly(thio)ethers via ring-opening polymerization. P1 and P2 denote poly(ethylene oxide) (PEO) and poly(ethylene sulfide) (PES), respectively
Synthesis
The synthesis of 2,2,6,6-Tetramethylpiperidinooxy involves several chemical steps, and its versatility makes it a valuable compound in various applications. Here is a simplified overview of the synthetic process: Starting Material: The synthesis often begins with a suitable precursor, such as 2,2,6,6-tetramethylpiperidine. This compound provides the basic piperidine structure necessary for 2,2,6,6-Tetramethylpiperidinooxy synthesis. Oxidation Reaction: The key step involves the oxidation of the precursor to introduce the nitroxyl moiety. This is typically achieved by using an oxidizing agent such as sodium hypochlorite (NaClO). The reaction results in the conversion of the piperidine ring to the nitroxyl radical, creating 2,2,6,6-Tetramethylpiperidinooxy. Purification: The synthesized 2,2,6,6-Tetramethylpiperidinooxy is then purified to remove any impurities, ensuring a high level of chemical purity. Purification methods may include techniques like column chromatography or recrystallization. Derivatization (Optional): Depending on the intended application, 2,2,6,6-Tetramethylpiperidinooxy can undergo derivatization to produce different 2,2,6,6-Tetramethylpiperidinooxy derivatives with specific properties. Derivatization may involve attaching functional groups to the 2,2,6,6-Tetramethylpiperidinooxy structure.The choice of specific reagents, reaction conditions, and purification methods can vary depending on the synthetic route chosen by chemists. It's important to note that the synthesis of 2,2,6,6-Tetramethylpiperidinooxy should be conducted with attention to safety protocols, given the potentially hazardous nature of some of the chemical reactions involved. Industrial-scale production of 2,2,6,6-Tetramethylpiperidinooxy is typically carried out with efficiency and precision, ensuring a stable supply for various applications. The compound's synthetic versatility and widespread use in both academic and industrial settings underscore its importance in the field of organic chemistry.
Safety
2,2,6,6-Tetramethylpiperidinooxy is generally considered safe when handled and used with proper precautions. However, like many chemical compounds, it poses potential risks, and adherence to safety guidelines is essential. Toxicity: 2,2,6,6-Tetramethylpiperidinooxy itself is not highly toxic, but exposure to large amounts or prolonged contact can cause irritation. Inhalation of 2,2,6,6-Tetramethylpiperidinooxy dust or vapors should be avoided. Standard laboratory safety measures, such as wearing appropriate personal protective equipment (PPE), are crucial. Skin and Eye Contact: Direct contact with the skin or eyes should be prevented. In case of contact, affected areas should be promptly washed with copious amounts of water. Safety goggles and gloves should be worn when handling 2,2,6,6-Tetramethylpiperidinooxy. Inhalation: 2,2,6,6-Tetramethylpiperidinooxy should be used in well-ventilated areas to minimize inhalation exposure. If working with larger quantities or in confined spaces, respiratory protection may be necessary. Fire Hazard: 2,2,6,6-Tetramethylpiperidinooxy is a stable compound, but, like many organic substances, it may pose a fire hazard under certain conditions. It is advisable to store 2,2,6,6-Tetramethylpiperidinooxy away from heat sources, open flames, and incompatible materials. Storage: Proper storage is crucial for maintaining the stability of 2,2,6,6-Tetramethylpiperidinooxy. It should be stored in a cool, dry place away from direct sunlight. Additionally, it should be kept away from reducing agents, acids, and strong oxidizing agents. Chemical Incompatibilities: 2,2,6,6-Tetramethylpiperidinooxy should not be mixed with incompatible substances, and its compatibility with other reagents and solvents should be considered during handling and storage. Emergency Measures: Knowledge of emergency measures, such as eye wash stations and emergency showers, is essential in case of accidental exposure. Emergency contact information for medical assistance should be readily available. Regulatory Compliance: Users should be aware of and comply with local, national, and international regulations regarding the handling, storage, and disposal of 2,2,6,6-Tetramethylpiperidinooxy. This includes waste disposal in accordance with applicable regulations. Researchers and professionals working with 2,2,6,6-Tetramethylpiperidinooxy should be familiar with its Material Safety Data Sheet or Safety Data Sheet, which provides detailed information on its properties, hazards, and safe handling practices. Additionally, consultation with institutional safety guidelines is crucial to ensure compliance with specific laboratory protocols.
Reference
1. Gerig JT, Reinheimer JD, Robinson RH. Modification of human serum albumin with N-(2,5-dinitro-4-fluorophenyl)-4-amino-2,2,6,6-tetramethyl-piperidinooxy radical. Biochim Biophys Acta. 1979 Aug 28;579(2):409-20.
2. Hatakeyama-Sato K, Wakamatsu H, Matsumoto S, Sadakuni K, Matsuoka K, Nagatsuka T, Oyaizu K. TEMPO-Substituted Poly (ethylene sulfide) for Solid-State Electro-Chemical Charge Storage. Macromol Rapid Commun. 2021 Feb;42(4):e2000607.
Related articles And Qustion
See also
Lastest Price from TEMPO manufacturers
US $0.00/kg2025-10-21
- CAS:
- 2564-83-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons
US $1.50/g2025-06-25
- CAS:
- 2564-83-2
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 10 Tons