ABT 263: pharmacokinetic, activities and clinical applications
General Description
ABT 263 is a potent orally bioavailable inhibitor of Bcl-2 family members, which are associated with tumor maintenance, progression, and chemoresistance. Its selectivity for Bcl-2 makes it a promising treatment option for BCL-2-dependent hematological malignancies, including CLL and AML. ABT 263 has also shown potential in solid tumors, such as SCLC and ovarian cancer. However, dose-dependent thrombocytopenia is a common side effect that requires careful management. Ongoing clinical trials aim to explore the potential of ABT 263 in combination with other treatment modalities to improve patient outcomes in various cancer types.

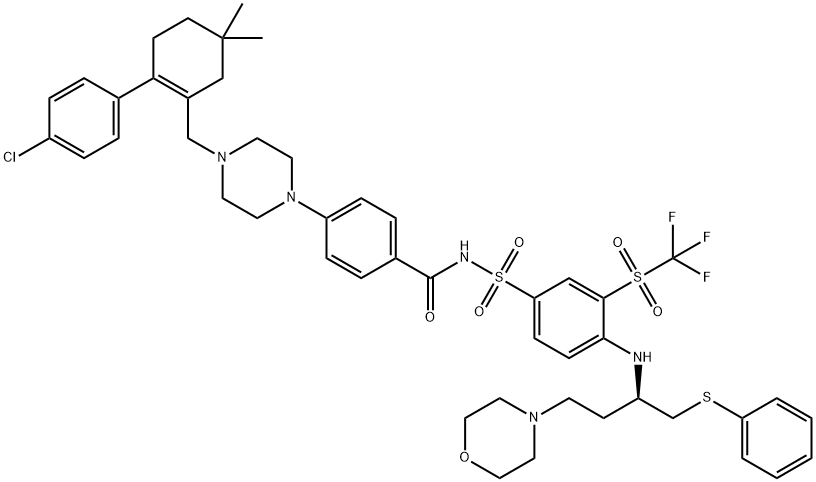
Figure 1. ABT 263
Pharmacokinetic
ABT 263 is a potent inhibitor of prosurvival Bcl-2 family members, such as Bcl-2, Bcl-xL, and Mcl-1, which are commonly associated with tumor maintenance, progression, and chemoresistance. While its predecessor, ABT-737, was not orally bioavailable, limiting its therapeutic potential, ABT 263 overcomes this limitation as a potent, orally bioavailable Bad-like BH3 mimetic with Ki values of less than 1 nmol/L for Bcl-2, Bcl-xL, and Bcl-w. In preclinical animal models, the oral bioavailability of ABT 263 ranges from 20% to 50%, depending on the formulation. ABT 263 disrupts Bcl-2/Bcl-xL interactions with pro-death proteins, leading to apoptosis initiation within 2 hours post-treatment in human tumor cells. It induces complete tumor regressions in xenograft models of small-cell lung cancer and acute lymphoblastic leukemia when administered orally alone. Furthermore, it enhances the efficacy of clinically relevant therapeutic regimens in aggressive B-cell lymphoma and multiple myeloma xenograft models. These findings support the rationale for clinical trials evaluating ABT 263 in small-cell lung cancer and B-cell malignancies. The oral efficacy of ABT 263 allows for dosing flexibility, maximizing its clinical utility as a single agent and in combination regimens. 1
Activities
ABT 263, also known as navitoclax, is a potent inhibitor of the B cell CLL/lymphoma 2 (BCL-2) family of proteins, which play a crucial role in regulating the process of apoptosis. This family consists of both proapoptotic and prosurvival proteins, and an imbalance in favor of the prosurvival proteins can enable cancer cells to evade apoptosis. While the development of ABT 263 demonstrated the therapeutic potential of directly inhibiting prosurvival proteins, its inhibition of both BCL-2 and BCL-2-like 1 (BCL-X(L)) led to on-target thrombocytopenia, limiting its efficacy. In response to this limitation, ABT 263 was re-engineered from ABT 263 to create a highly potent, orally bioavailable, and BCL-2-selective inhibitor. This compound has been shown to effectively inhibit the growth of BCL-2-dependent tumors in vivo while sparing human platelets. Clinical studies have demonstrated the promising potential of ABT 263 in the treatment of BCL-2-dependent hematological cancers, with evidence of rapid tumor lysis within 24 hours of administration in patients with refractory chronic lymphocytic leukemia. These findings highlight the selective pharmacological inhibition of BCL-2 as a promising avenue for the targeted treatment of BCL-2-dependent hematological cancers. 2
Clinical applications
ABT 263 is a small molecule inhibitor that has shown promising clinical applications in the treatment of various cancers. It works by targeting and inhibiting the anti-apoptotic proteins Bcl-2, Bcl-xL, and Bcl-w, which play a critical role in preventing programmed cell death (apoptosis) in cancer cells. One of the primary clinical applications of ABT 263 is in hematological malignancies, such as chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML). In CLL patients, ABT 263 has demonstrated significant efficacy, particularly in individuals with high-risk genetic abnormalities. Similarly, in AML patients, ABT 263 has shown potential to enhance the effectiveness of conventional chemotherapy regimens. ABT 263 has also been investigated in solid tumors, including small cell lung cancer (SCLC) and ovarian cancer. In SCLC, ABT 263 has exhibited promising activity when used in combination with standard chemotherapeutic agents. Furthermore, preclinical studies have suggested that ABT 263 may sensitize ovarian cancer cells to platinum-based chemotherapy. Despite its potential, ABT 263 does have limitations, including dose-dependent thrombocytopenia (reduced blood platelet count) as a common side effect. Therefore, careful monitoring and management of this adverse event are necessary during treatment. In conclusion, ABT 263 holds promise as a targeted therapy for hematological malignancies and certain solid tumors. Ongoing clinical trials aim to further explore its potential in combination with other treatment modalities, with the ultimate goal of improving patient outcomes in various cancer types. 3
Reference
1. Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421-3428.
2. Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202-208.
3. Yang IH, Jung JY, Kim SH, et al. ABT-263 exhibits apoptosis-inducing potential in oral cancer cells by targeting C/EBP-homologous protein. Cell Oncol (Dordr). 2019;42(3):357-368.