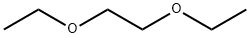Examples of research on Ethylene glycol diethyl ether
Description
Ethylene glycol diethyl ether, also called 3,6-dioxaoctane or 1,2-Diethoxyethane (1,2-DEE), appears as a clear colorless liquid with a faint ether-like odor. The flash point is 95 °F. Less dense than water and insoluble in water. In addition, it is vapors are heavier than air. It is an important solvent and extractant widely used for producing nitrocellulose, rubber, and resin. It has excellent solubility to many organic compounds such as ethanol, acetone, and toluene[1].
Caes 1
Sarkar et al. studied the reaction pathways for oxygenates, acetic acid, ethylene glycol (EG), 2-ethoxyethanol (2-EE), and 1,2-diethoxyethane (1,2-DEE) added during Fischer–Tropsch synthesis (FTS) over a doubly promoted fused iron catalysts. They found that the addition of acetic acid, EG, and 2-EE affected only slightly the CO conversion but resulted in a significant reduction in H 2 conversion. In contrast, adding 1,2-DEE resulted in a slight increase in H2 and CO conversion. Added acetic acid reversibly increased the CO2 production rate, while EG, 2-EE, and 1,2-DEE decreased the CO2 selectivity. The addition of these oxygenates reduced the production rate of methane. The addition of acetic acid and 1,2-DEE decreased methanol selectivity significantly while adding EG resulted in a significant increase in methanol production[2]. The results suggest that acetic acid undergoes some C-C bond rupture while 2-EE and 1,2-DEE undergo cleavage of the ether linkage (C-O-C bond).
Caes 2
The densities and ultrasonic speeds of binary mixtures of Ethylene glycol diethyl ether with n-heptane were measured at 298.15 K by Kumaran et al. The excess volumes calculated from their density data are positive at all solution compositions and interpreted using the Flory theory, whereas, according to the report from Fuene, the excess volumes of binary mixtures of 1,2-DEE with n-propanol are harmful as a result of the formation of hydrogen bonds between the -OH group of n-propanol and the -O- atom of 1,2-DEE. In 2017, Long et al. measured the densities and surface tensions of a binary mixture of 1,2-dimethoxyethane (DEE) and toluene over the entire composition at four temperatures of 293.15, 303.15, 313.15, and 323.15 K and atmospheric pressure. The excess molar volumes VE of the binary mixture are negative, which verifies the occurrence of volume contraction during the mixing of DEE and toluene[3].
Caes 3
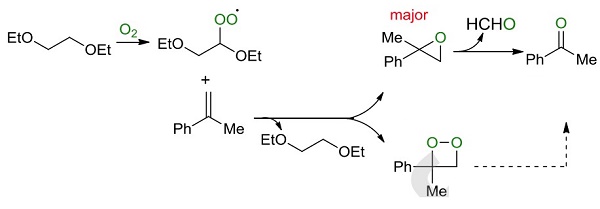
Ethylene glycol diethyl ether could be employed as a catalyst and ambient air as an oxidant to achieve an efficient protocol for the construction of various aryl-alkyl and diaryl ketones through oxidative cleavage of gem-disubstituted aromatic alkenes under minimal solvent conditions[1]. The results manifested that the Ethylene glycol diethyl ether not only served as a reaction medium and acted as a catalyst in the developed oxidation. 1,2-diethoxyethane could be used as a starting material to synthesize acetophenone. Firstly, 1,2-diethoxyethane reacted with dioxygen under heating conditions to produce a peroxyl radical A, which oxidized α-methyl styrene 1a to form 2-methyl-2-phenyloxirane 3a and regenerate the 1,2-diethoxyethane. Finally, the decomposition of 3a afforded the desired product acetophenone and HCHO by-product regeneration.
References
[1] Kai-Jian Liu . “1,2-Diethoxyethane catalyzed oxidative cleavage of gem-disubstituted aromatic alkenes to ketones under minimal solvent conditions.” Chinese Chemical Letters 31 7 (2020): Pages 1868-1872.
[2] Amitava Sarkar . “Fischer–Tropsch synthesis with promoted iron catalyst: Reaction pathways for acetic acid, glycol, 2-ethoxyethanol and 1,2-diethoxyethane.” Applied Catalysis A: General 341 1 (2008): Pages 146-153.
[3] Bingwen Long. “Intermolecular interactions of 1,2-diethoxyethane with toluene: An insight from surface and volumetric properties at different temperatures.” Journal of Molecular Liquids 249 (2018): Pages 1-8.
See also
Lastest Price from Ethylene glycol diethyl ether manufacturers
US $0.00/KG2024-03-27
- CAS:
- 629-14-1
- Min. Order:
- 180KG
- Purity:
- 99.0%MIN
- Supply Ability:
- 3000tons