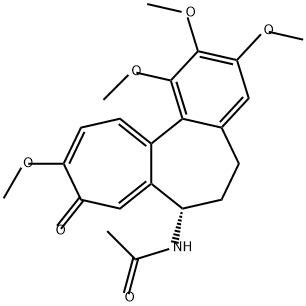
Total synthesis of colchicine
Colchicine has been isolated from, inter uliu, the meadow saffron Colchicum autumnale and might well be described as the prototypic anti-mitotic drug although clinical applications of this alkaloid are restricted because of its toxicity.
Banwell 1996 total synthesis of colchicine
The useful biological properties and novel structure of this compound has resulted in considerable effort being directed towards its synthesis. It has been suggested that the troponoid C-ring of colchicine is formed late in the biosynthetic process, possibly by the route illustrated in Scheme 1. This suggestion prompted us to examine whether elements of such a pathway could be mimicked in the laboratory thus permitting development of what would be the first regio- and enantio- controlled total synthesis of (1). Such an approach has been very fruitful.
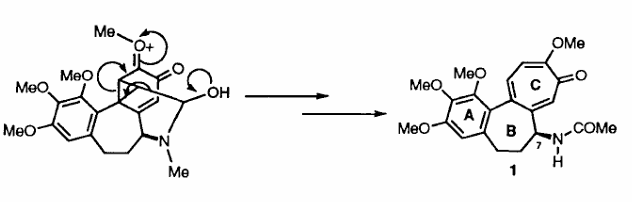
Scheme 1: Late Stages in the Biosynthesis of Colchicine (1)
Our synthesis (Scheme 2) begins with benzaldehyde (2) and acetophenone (3) which are readily elaborated, via an initial Claisen-Schmidt condensation reaction, to the 1.3-diarylpropanol (4). Subjection of this last compound to an oxidative coupling protocol developed by Umezawa7 ultimately afforded the dibenzocycloheptenone (5). In anticipation of the enantioselective introduction of the C-7 acetamido group associated with (1) it was necessary, for various reasons, to form alcohol (6) with the R- configuration at C-7 (colchicine numbering). To these ends, the ketone (5) was subjected to enantioselective reduction with stoichiometric quantities of the CBS-reagent8 and, after debenzylation, the target compound (6) was obtained in 94% ee. Taylor-McKillop oxidation9 of this phenol followed by nucleophilic c yclopropanation of the derived cyclohexadienone (7) with dimethylsulfoxonium methylide then provided the key a-homo-o-benzoquinone mono-acetal(8) (98% ee after one recrystallisation), the structure (including absolute configuration) of which was established by single-crystal X-ray analysis.[1]
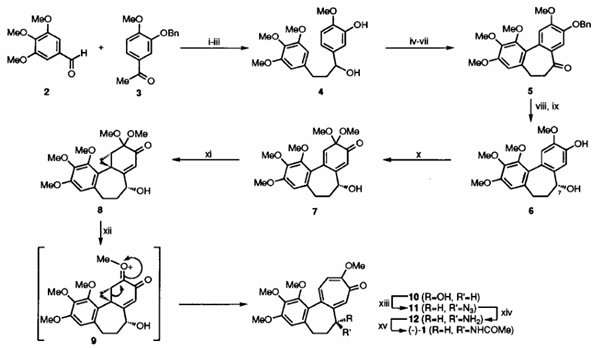
Scheme 2: Total Synthesis of (-)-Colchicine (1)
Reagents and conditions: (i) NaOH, MeOH, RT, 48h, 96%; (ii) H2, Pd on C, MeC02Et, 15OC, 10h, 96%; (iii) NaBb, W/MeOH, 15"C, lSh, 96%; (iv) Pb(OCOMe)q, 3A sieves, CH2C12, 15°C lh, 100%; (v) CF~COZH, 3A sieves, THF/C&j, O'C, lh, 42%; (vi) BnBr, K2CO3, MeCN, 82"C, 4h, 88%; (vii) NMO, "PAP, 4A sieves, CH2C12, 15OC, 43h, 98%; (viii) CBS-reagent, THF, 15OC. 6h, 88%; (ix) H2. Pd on C. MeC@Et, 15OC, 9h, 99%; (x) m(No3)3, MeOH, -2OoC, OSh, 83%; (xi) Me3S(O)I, NaH, DMSO, 15'C, 7h, 54% @ 82% conversion; (xii) CF3CO2H. CH2C12, 15OC, 3h, 48%; (xiii) i-Pr02CN=NC02Pr-i, PPh3, Zn(N3)2-2(CgHgN), THF, 15"C, 38h, 30%; (xiv) PPh3. H20, THF, 15OC, 63h; (xv) (MeC0)20, CgHgN, 15°C. 0.25h, 60% from 11.
Reaction of compound (8) with trifluoroacetic acid in dichloromethane resulted in the desired (biomimetic) ring-expansion and formation of troponoid (10) (98% ee). Most likely, this key conversion proceeds via the oxonium ion (9). Mitsunobu chemistryll was used to effect sN2 displacement of the hydroxy group in compound (10) by azide ion. The resulting azido-compound (11) (> 95% ee) was subjected to reduction under Staudinger conditions and the amine (12) so-formed was immediately acetylated thereby affording colchicine (1) (> 81%ee).
References
[1] Banwell, M. “Cyclopropyl compounds as chemical building blocks: Total syntheses of the alkaloids (-)-colchicine, imerubrine and grandirubrine.” Pure and Applied Chemistry 68 1 (1996): 539–542.
Related articles And Qustion
Lastest Price from Colchicine manufacturers

US $0.00/kg2025-04-27
- CAS:
- 64-86-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00/KG2025-04-21
- CAS:
- 64-86-8
- Min. Order:
- 1KG
- Purity:
- ≥98% HPLC
- Supply Ability:
- 1000KG