Properties and Synthesis of Lithium triisobutylhydroborate
Lithium triisobutylhydroborate (L-selectride) is an organoborane compound that functions as a specialized reducing agent in organic chemistry, notably employed for ketone reduction as a key step in the Overman strychnine synthesis. The distinctive steric properties of Lithium triisobutylhydroborate contribute to its exceptional stereoselectivity in complex molecular transformations.

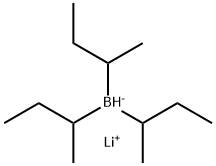
Figure1: Picture of Lithium triisobutylhydroborate
General Description
Lithium triisobutylhydroborate, commercially recognized as Lithium tri-sec-butylborohydride or L-Selectride, represents a highly efficient reducing agent widely employed in organic synthesis, characterized by the chemical formula C12H28BLi and a molecular weight of 190.11 g/mol (CAS Registry Number: 38721-52-7). Typically supplied as a standardized 1.0 M tetrahydrofuran (THF) solution, Lithium triisobutylhydroborate has become the reagent of choice for achieving precise stereoselective reduction outcomes in complex molecular transformations. As China's premier manufacturer of this specialized compound, we guarantee superior product quality meticulously engineered to meet the rigorous demands of both industrial-scale applications and advanced research initiatives.
Properties
Lithium triisobutylhydroborate typically presents as a transparent, colorless liquid formulation with a measured density of approximately 0.89 g/mL at standard room temperature (25°C), while exhibiting a critically low flash point of 1°F (-17°C) that categorizes it as highly flammable material. This organoborane compound demonstrates extreme sensitivity to atmospheric oxygen and moisture, undergoing rapid hydrolysis upon water contact with concomitant release of combustible gaseous products. Although precise melting and boiling points remain unspecified in literature due to its predominant commercial distribution in stabilized THF solutions, Lithium triisobutylhydroborate maintains structural integrity when stored under inert atmospheric conditions such as argon encapsulation. Its distinctive solubility behavior manifests through vigorous reactions with protic solvents, necessitating stringent handling protocols and specialized storage requirements. Comparative analysis with NaBH₄ reveals that the reaction selectivity of L-Selectride primarily stems from two structural features: (1) its molecular structure contains only one hydride ion, enabling precise stoichiometric control, and (2) the presence of three isobutyl groups creates significant steric hindrance, favoring selective reactions at less hindered sites of substrates. Furthermore, the reducing capability of L-Selectride is markedly influenced by reaction temperature. In THF solvent, ketone reduction proceeds efficiently at low temperatures, allowing excellent compatibility with various sensitive functional groups including cyano, ketal, acetal, and silyl ether moieties without observable degradation.
Synthesis
A synthetic methodology for Lithium triisobutylhydroborate has been documented in scientific literature, involving sequential procedures commencing with the preparation of isobutyl bromide diethyl ether solution by blending isobutyl bromide with anhydrous diethyl ether. The reaction system is established in a specialized vessel containing magnesium powder, boron trifluoride etherate and anhydrous diethyl ether under continuous agitation, followed by controlled addition of the isobutyl bromide solution to initiate the Grignard-type reaction. After stationary phase separation, the supernatant undergoes distillation to obtain triisobutylborane intermediate, which is subsequently dissolved in organic solvent and treated with catalytic species—selectively chosen from 1,4-diazabicyclo[2.2.2]octane (DABCO) or novel diazacyclic compounds synthesized from 2-amino-1,3-benzodioxole-5-ethanol—prior to the dropwise introduction of lithium aluminum hydride solution under nitrogen atmosphere and ice-bath conditions. The optimized preparation of Lithium triisobutylhydroborate through this catalytic protocol ultimately yields the target product in substantially enhanced isolated yield after filtration, demonstrating significant improvement over conventional synthetic routes. [1]
Application
Lithium triisobutylhydroborate primarily serves as an organic synthetic intermediate, functioning as an excellent, mild, and highly selective reducing agent in organic transformations. As a representative member of the Selectride family, this reagent demonstrates superior solubility and cost-effectiveness, leading to its widespread application in synthetic chemistry. Lithium triisobutylhydroborate has gained extensive adoption as a specialized reducing agent in sophisticated organic transformations, particularly valued for its exceptional regio- and stereoselectivity profiles that enable precise molecular editing. This compound demonstrates remarkable versatility across multiple domains, including enantioselective amino acid synthesis, diastereoselective reduction protocols, and oxygen heterocycle construction, establishing Lithium triisobutylhydroborate as an indispensable tool for both pharmaceutical intermediate manufacturing and cutting-edge academic investigations. The potent reducing capabilities of this reagent render it particularly suitable for precision chemical processes, making our enterprise the preferred Lithium triisobutylhydroborate supplier for Chinese researchers and industrial practitioners requiring guaranteed reagent performance in critical synthetic applications.
Reference
[1] Qiao, H. Y. A Preparation Method for Lithium triisobutylhydroborate: CN202211577000.9[P].
Lastest Price from Lithium triisobutylhydroborate manufacturers
US $1.10-9.90/kg2025-08-18
- CAS:
- 38721-52-7
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 100kg

US $4.00/kg2025-04-21
- CAS:
- 38721-52-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000