-
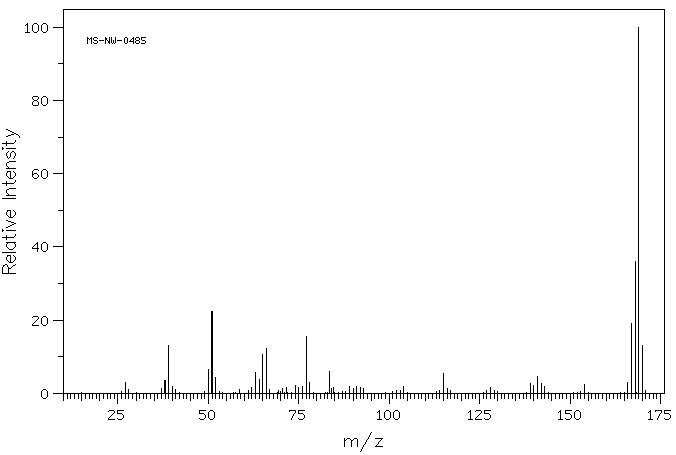
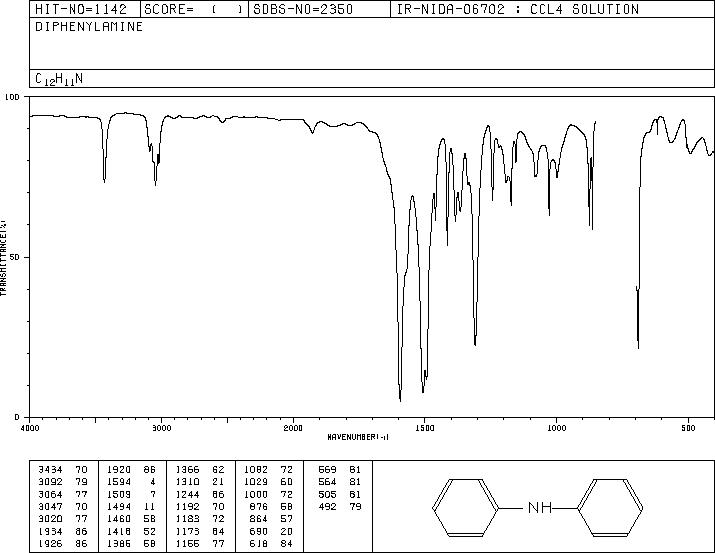
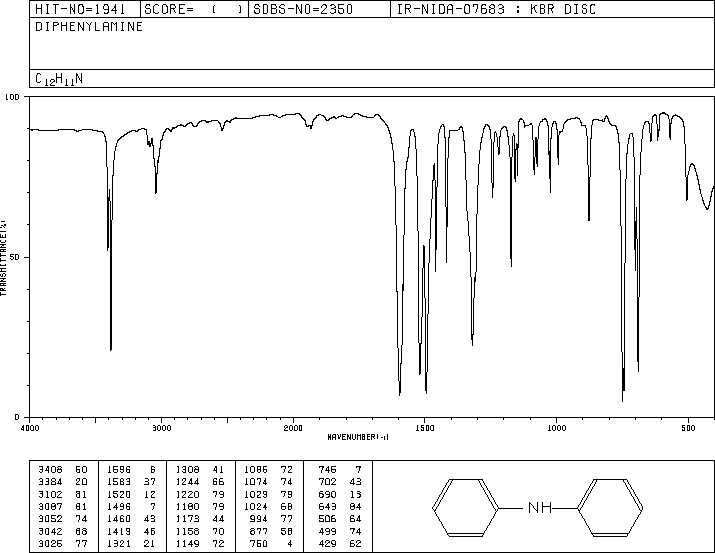
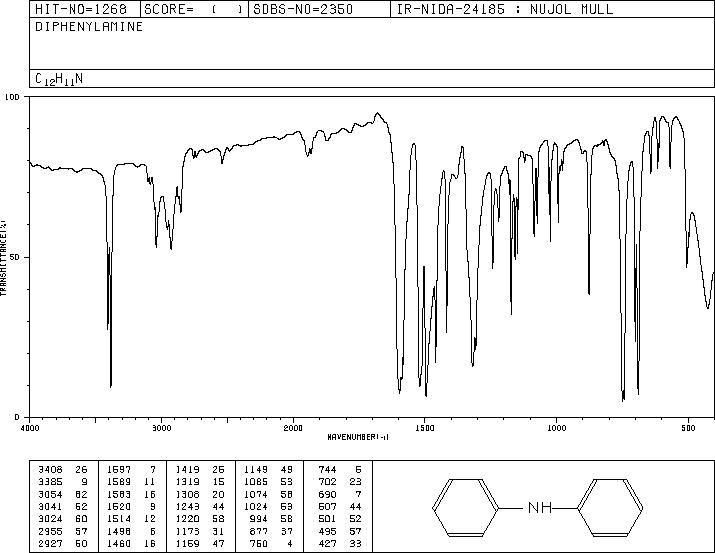
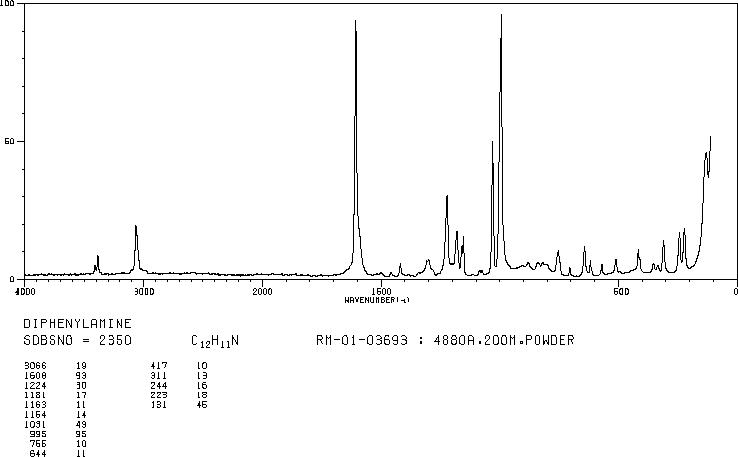
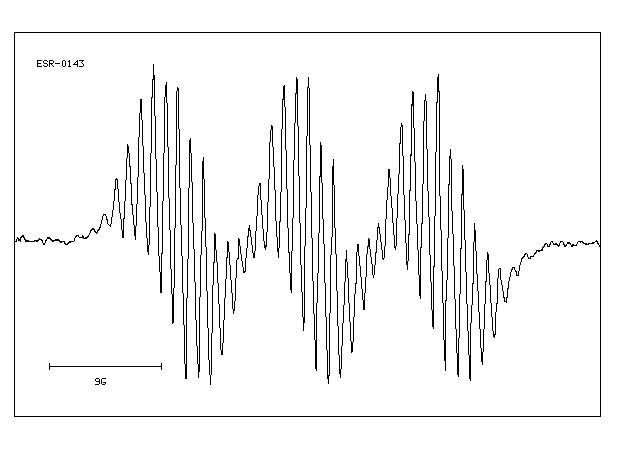
MS-NW-0485 diphenylamine C12H11N (Mass of molecular ion: 169)
Source Temperature: 150 °C Sample Temperature: 120 °C Direct, 75 eV
27.0 3.0 28.0 1.1 37.0 1.2 38.0 3.6 39.0 13.2 40.0 1.9 41.0 1.1 50.0 6.6 51.0 22.5 52.0 4.4 58.5 1.0 62.0 1.6 63.0 5.6 64.0 3.8 65.0 10.6 66.0 12.2 67.0 1.0 70.5 1.3 71.5 1.6 74.0 2.1 75.0 1.7 76.0 1.8 77.0 15.4 78.0 2.9 83.5 5.9 84.0 1.3 84.5 1.7 89.0 2.0 90.0 1.3 91.0 2.0 92.0 1.7 93.0 1.2 104.0 2.0 115.0 5.5 116.0 1.3 128.0 1.5 139.0 2.7 140.0 2.2 141.0 4.5 142.0 2.8 143.0 1.8 154.0 2.5 166.0 3.1 167.0 19.1 168.0 35.9 169.0 100.0 170.0 13.1
in DMSO-d6
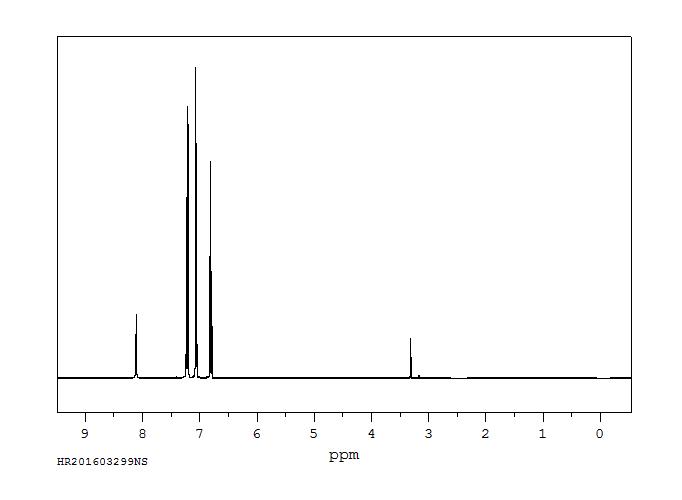
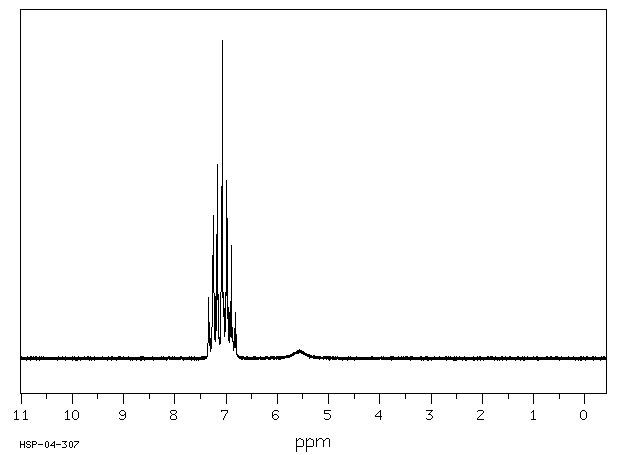
400 MHz in CDCl3
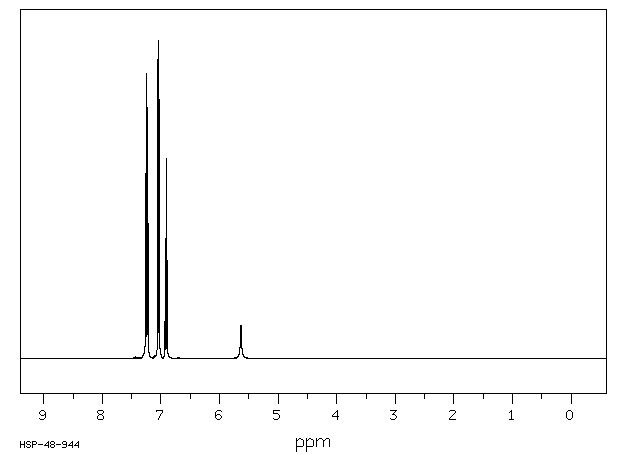
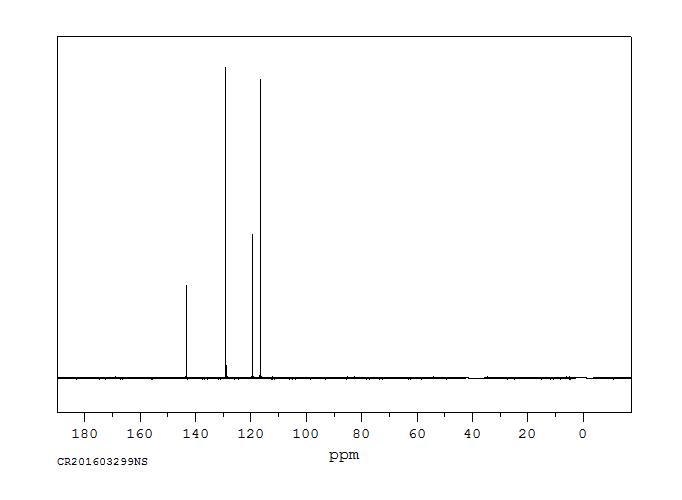
90 MHz in CDCl3
-
1H NMR 399.65 MHz C12 H11 N 0.040 g : 0.5 ml CDCl3 diphenylamine 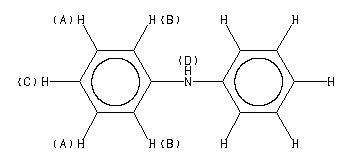
Assign. Shift(ppm)
A 7.241 B 7.041 C 6.910 D 5.63
Hz ppm Int.2906.74 7.274 37 2904.42 7.268 112 2902.34 7.263 581 2900.39 7.258 231 2897.22 7.250 163 2895.02 7.244 843 2893.80 7.241 897 2891.85 7.236 169 2888.43 7.228 293 2886.47 7.223 790 2884.40 7.218 131 2820.68 7.058 196 2818.85 7.054 836 2817.63 7.051 1000 2815.55 7.046 297 2812.13 7.037 301 2811.16 7.035 490 2810.18 7.032 814 2809.08 7.029 721 2806.76 7.024 117 2769.65 6.931 228 2768.55 6.928 377 2767.46 6.925 209 2762.21 6.912 324 2761.23 6.910 631 2760.25 6.907 310 2757.32 6.900 35 2755.00 6.894 191 2753.91 6.891 307 2752.69 6.888 170 2251.59 5.634 104
-
1H NMR 89.56 MHz C12 H11 N 0.039 g : 0.5 ml CDCl3 diphenylamine 
Assign. Shift(ppm)
A 7.22 B 7.04 C 6.92 D 5.6
Hz ppm Int.658.06 7.348 123 657.00 7.336 193 655.19 7.316 105 650.81 7.267 323 649.44 7.252 451 648.31 7.239 406 647.25 7.228 158 644.56 7.197 194 642.44 7.174 609 642.00 7.169 517 641.63 7.165 539 640.25 7.149 202 637.81 7.122 50 635.00 7.091 543 633.63 7.075 839 633.13 7.070 1000 630.75 7.043 207 628.06 7.013 185 627.63 7.008 199 627.06 7.002 208 626.19 6.992 559 624.75 6.976 439 623.00 6.957 160 621.44 6.939 58 619.94 6.923 156 618.63 6.908 181 617.75 6.898 357 615.25 6.870 97 612.44 6.839 93 610.81 6.821 145 609.31 6.804 75
更多供应商