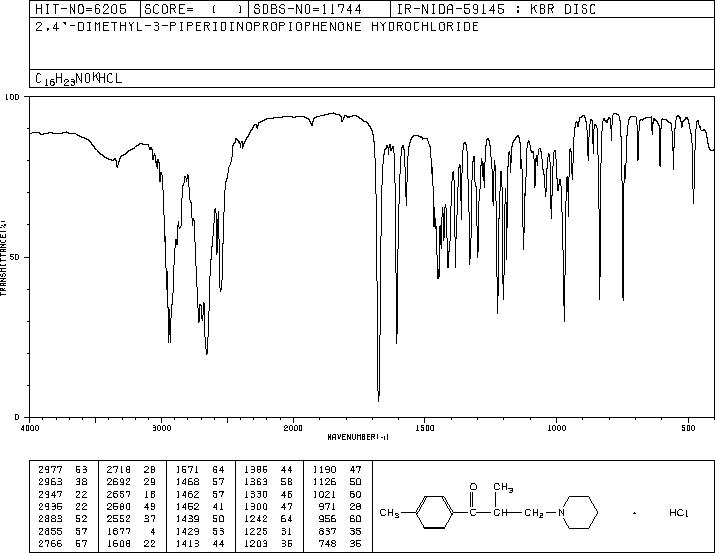
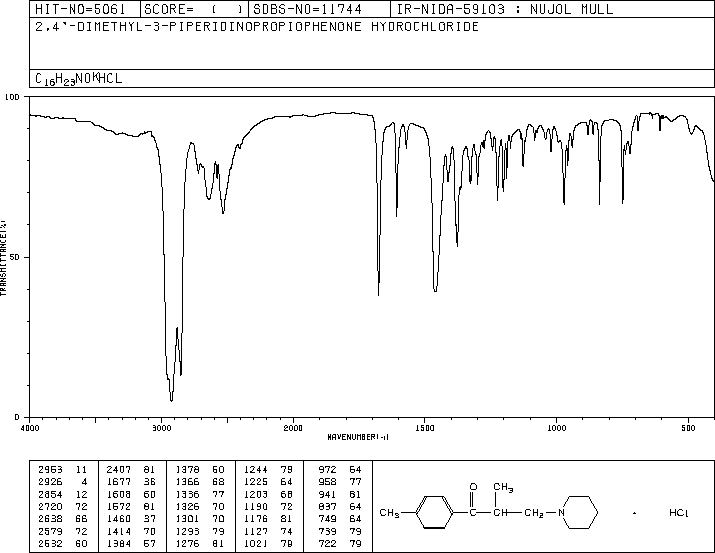
-
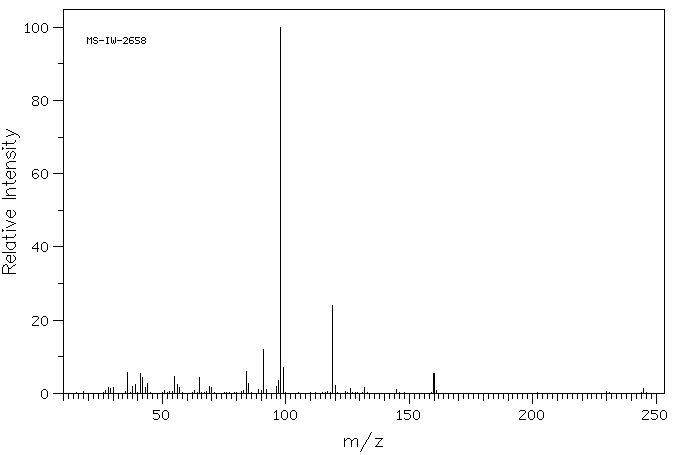
MS-IW-2658 2,4'-dimethyl-3-piperidinopropiophenone hydrochloride C16H23NO HCl (Mass of molecular ion: 245)
Source Temperature: 150 °C Sample Temperature: 120 °C Direct, 75 eV
28.0 1.6 29.0 1.4 30.0 1.5 36.0 5.8 38.0 2.0 39.0 2.5 41.0 5.5 42.0 4.3 43.0 1.5 44.0 2.7 55.0 4.5 56.0 2.5 57.0 1.7 65.0 4.3 69.0 1.8 70.0 1.7 84.0 5.9 85.0 2.8 89.0 1.0 91.0 12.0 92.0 1.0 96.0 1.9 97.0 3.5 98.0 100.0 99.0 7.1 119.0 24.1 120.0 2.1 126.0 1.3 132.0 1.6 145.0 1.0 160.0 5.5 245.0 1.2
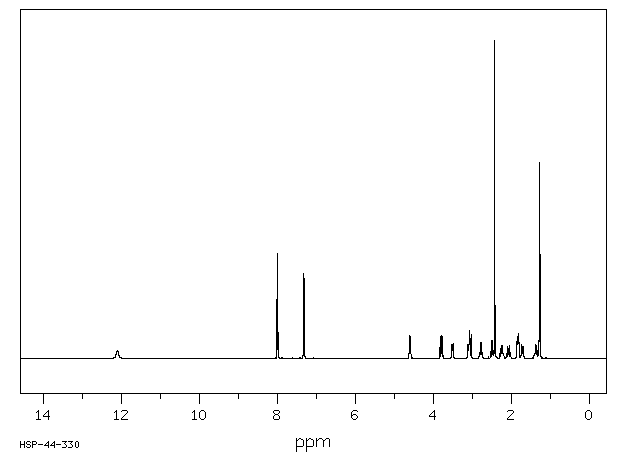
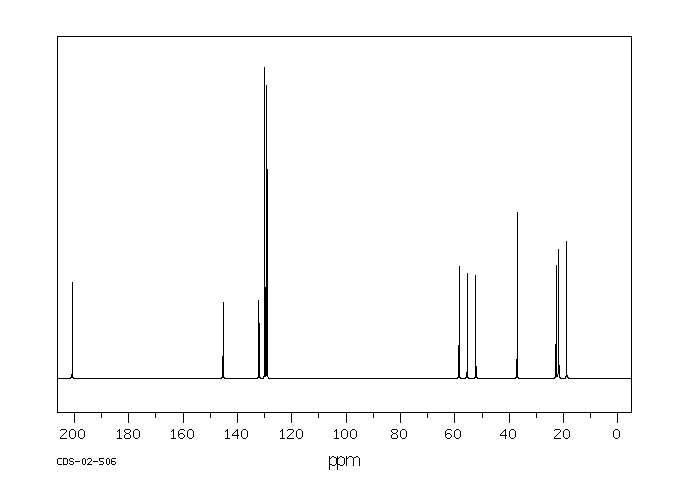
400 MHz in CDCl3
-
1H NMR 399.65 MHz C16 H23 N O 0.038 g : 0.5 ml CDCl3 2,4'-dimethyl-3-piperidinopropiophenone hydrochloride 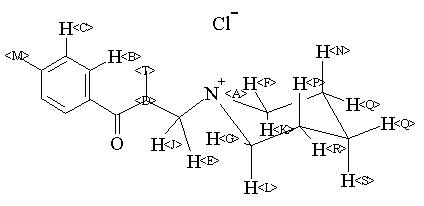
Assign. Shift(ppm)
A 12.1 B 8.006 C 7.325 D 4.610 E 3.806 F *1 3.519 G *1 3.107 J 3.062 K *2 2.791 L *2 2.502 M 2.437 N 2.256 P 2.086 Q 1.84 R 1.729 S 1.379 T 1.290 J(E,J)=-12.6HZ J(E,D)=8.6HZ J(F,K)=-12.4HZ J(G,L)=-12.3HZ J(D,J)=2.0HZ ASSIGNED BY H-H AND C-H COSY.
Hz ppm Int.3203.77 8.017 310 3197.17 8.000 115 3195.52 7.996 332 2931.58 7.336 267 2927.37 7.325 30 2923.52 7.316 253 2922.97 7.314 229 1851.63 4.634 37 1849.98 4.630 47 1842.84 4.612 72 1835.51 4.593 49 1834.05 4.590 41 1536.40 3.845 36 1527.79 3.823 68 1523.76 3.813 44 1519.18 3.802 40 1515.15 3.792 72 1506.54 3.770 37 1412.95 3.536 44 1412.58 3.535 44 1411.85 3.533 43 1400.86 3.506 48 1400.49 3.505 48 1248.83 3.125 44 1248.28 3.124 44 1236.74 3.095 51 1236.19 3.094 51 1231.25 3.081 69 1229.23 3.076 87 1227.40 3.072 59 1218.42 3.049 52 1216.41 3.044 75 1121.89 2.808 49 1118.96 2.800 38 1112.74 2.785 38 1109.62 2.777 51 1006.68 2.519 56 1003.57 2.512 40 997.34 2.496 41 994.41 2.489 58 974.08 2.438 1000 909.79 2.277 40 908.51 2.274 32 907.96 2.272 32 898.80 2.249 30 894.95 2.240 39 841.10 2.105 39 839.82 2.102 31 839.45 2.101 30 828.46 2.073 31 826.45 2.068 42 744.94 1.864 69 742.56 1.859 69 736.51 1.843 47 731.57 1.831 78 731.20 1.830 77 727.72 1.821 62 698.78 1.749 45 696.95 1.744 43 684.31 1.713 38 682.30 1.708 36 557.38 1.395 44 547.85 1.371 32 544.37 1.363 40 519.46 1.300 618 512.13 1.282 610
More Suppliers