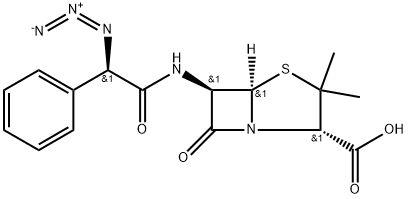
AZIDOCILLIN
- Product NameAZIDOCILLIN
- CAS17243-38-8
- MFC16H17N5O4S
- MW375.4
- EINECS241-278-5
- MOL File17243-38-8.mol
Usage And Synthesis
Example 1: α-Azidobenzylpenicillin via the Mixed Anhydride - A solution of α-
azidophenylacetic acid (8.9 grams, 0.05 mol) of triethylamine (5.1 grams,
0.05 mol) in 50 ml of dry dimethylformamide was stirred and chilled below -
5°C. At this temperature ethyl chloroformate (4.7 ml) was added in portions
so that the temperature was never above -5°C. After the mixture had been
stirred for 20 minutes, dry acetone (100 ml), chilled to -5°C, was added in
one portion, immediately followed by an ice-cold solution of 6-
aminopenicillanic acid (10.8 grams, 0.05 mol) and triethylamine (5.1 grams,
0.05 mol) in 100 ml of water, and the stirring was continued for 1? hours at
0°C.
The pH of the mixture was adjusted to 7.5 by adding a saturated sodium bicarbonate solution. After being washed twice with diethyl ether, the reaction solution was acidified to pH 2 with dilute hydrochloric acid and extracted with ether. The ether solution containing the free penicillin was washed twice with water and then extracted with 50 ml of N potassium bicarbonate solution. After freeze drying of the obtained neutral solution, the potassium salt of α- azidobenzylpenicillin was obtained as a slightly colored powder (11.2 grams, 54% yield) with a purity of 55% as determined by the hydroxylamine method (the potassium salt of penicillin G being used as a standard).
The infrared spectrum of this substance showed the presence of an azido group and a beta-lactam system. The substance inhibited the growth of Staph. aureus Oxford at a concentration of 0.25 mcg/ml.
Example 2: α-Azidobenzylpenicillin via the Acid Chloride - 6-aminopenicillanic acid (18.5 grams, 0.085 mol) and sodium bicarbonate (21 grams, 0.025 mol) were dissolved in 200 ml of water and 100 ml of acetone. To this solution, chilled in ice, was added α-azidophenylacetyl chloride (16.6 grams, 0.085 mol), diluted with 10 ml of dry acetone. The temperature is held at 0° to 5°C and the reaction mixture was stirred for 2? hours.
The resulting solution was treated as described in Example 1 to give the potassium salt of α-azidobenzylpenicillin as a white powder (29.4 grams, 84% yield) with a purity of 83% as determined by the hydroxylamine method (the potassium salt of penicillin G being used as a standard).
The product showed the same properties as the product obtained in Example 1,. it inhibits the growth of Staph. aureus Oxford at a concentration of 0.13 mcg/ml.
The α-azidophenylacetyl chloride was prepared by treating α-azidophenylacetic acid with thionylchloride in portions at room temperature and then heating the solution under reflux for one hour. The α-azidophenylacetyl chloride distils at 115°C under a pressure of 10 mm Hg.
The pH of the mixture was adjusted to 7.5 by adding a saturated sodium bicarbonate solution. After being washed twice with diethyl ether, the reaction solution was acidified to pH 2 with dilute hydrochloric acid and extracted with ether. The ether solution containing the free penicillin was washed twice with water and then extracted with 50 ml of N potassium bicarbonate solution. After freeze drying of the obtained neutral solution, the potassium salt of α- azidobenzylpenicillin was obtained as a slightly colored powder (11.2 grams, 54% yield) with a purity of 55% as determined by the hydroxylamine method (the potassium salt of penicillin G being used as a standard).
The infrared spectrum of this substance showed the presence of an azido group and a beta-lactam system. The substance inhibited the growth of Staph. aureus Oxford at a concentration of 0.25 mcg/ml.
Example 2: α-Azidobenzylpenicillin via the Acid Chloride - 6-aminopenicillanic acid (18.5 grams, 0.085 mol) and sodium bicarbonate (21 grams, 0.025 mol) were dissolved in 200 ml of water and 100 ml of acetone. To this solution, chilled in ice, was added α-azidophenylacetyl chloride (16.6 grams, 0.085 mol), diluted with 10 ml of dry acetone. The temperature is held at 0° to 5°C and the reaction mixture was stirred for 2? hours.
The resulting solution was treated as described in Example 1 to give the potassium salt of α-azidobenzylpenicillin as a white powder (29.4 grams, 84% yield) with a purity of 83% as determined by the hydroxylamine method (the potassium salt of penicillin G being used as a standard).
The product showed the same properties as the product obtained in Example 1,. it inhibits the growth of Staph. aureus Oxford at a concentration of 0.13 mcg/ml.
The α-azidophenylacetyl chloride was prepared by treating α-azidophenylacetic acid with thionylchloride in portions at room temperature and then heating the solution under reflux for one hour. The α-azidophenylacetyl chloride distils at 115°C under a pressure of 10 mm Hg.
Preparation Products And Raw materials
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine