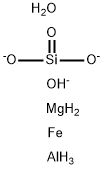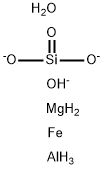Vermiculite is a clay mineral. It is similar to trioctahedral
smectites, with a structure that is balanced by
interlayer ions of mostly magnesium and sometimes
calcium. In other words, vermiculite is a hydrated
silicate, which results from the alteration of mica,
especially an aluminosilicate of magnesium. The formula
of vermiculite is:
Vermiculite is a tri-octahedral phyllite (2: 1) with a
basal spacing of lOA varying between 1A and lOA,
according to the nature of the interlayer cations.
The following are the principal differences between
vermiculites and smectites. (i) Vermiculites have a large
grain size. (ii) Vermiculites have a higher cation
exchange capacity and, when treated with glycol, achieve
basal spacing of about 14A while smectites take double
layers, with a basal spacing swelling to 17A. (iii)
Smectites dehydrate more rapidly while vermiculites
rehydrate more rapidly.
In soils, vermiculites are mostly clays of
transformation, formed from the degradation of micas or
illites. They have a high cation exchange capacity
(CEC) of 100 to 150 meq/100 g, which endows them a
high affity for weakly hydrated ions such as K+, N&+
and Caz+. Consequently, vermiculite-rich soils exhibit
high potassium-fixation capacity.
Vermiculite does not swell as much as
montmorillonite. Vermiculite is a 2:1 clay mineral,
similar in structure to hydrous magnesium. On rapid
heating, vermiculite assumes a worm-like shape, alluding
to a peculiar exfoliation phenomenon. When heated, it
can also expand to a lightweight, highly absorbent,
fireproof material about 16 times its original volume. In
natural state, the mineral has no useful application, but it
provides a low-density material with excellent thermal
and acoustic insulation properties when exfoliated.
Vermiculites in flakes are useful as a medium for
growing tissue-culture plants and for insulation.