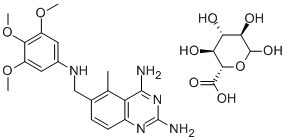
Trimetrexate glucuronate is a nonclassical antifolate introduced in the
U.S.A. and Canada as second-line therapy for the treatment of Pneumocystis carinii
pneumonia (PCP) and toxoplasmosis in AIDS patients who are refractory or
intolerant to co-trimoxazole therapy. Trimetrexate glucuronate is reportedly to be in
clinical trials for the treatment of several types of cancer including colorectal,
gastric, pancreas, prostate, bladder, lung, breast, head, and neck cancers. It is
also in early clinical trials for topical use in treating psoriasis and as an oral drug to
treat rheumatoid arthritis. The mechanism of action of trimetrexate glucuronate is
via inhibition of the enzyme dihydrofolate reductase which mediates the reduction of
dihydrofolate to tetrahydrofolic acid and results in disruption of DNA, RNA and
protein synthesis, with consequent cell death. Due to its toxicity to bone marrow
and the GI tract, trimetrexate glucuronate must be concurrently administered with
leucovorin as a rescue agent.
CI 898 Glucuronate is a dihydrofolate reductase (DHFR) inhibitor.
A mixture of 2,4-diamino-5-methyl-6-[(3,4,5-
trimethoxyanilino)methyl]quinazoline (1.0 g) and glucuronic acid (0.7 g) in
methanol (65 ml) is heated to dissolve the solid. The solution is cooled to
10°C and filtered to remove a small amount of solid. The filtrate is heated to
reflux and ethyl acetate is added to the cloud point. The warm solution is
filtered then slowly cooled. The solid that forms is collected, washed first with
ethylacetate, then with ether and dried in vacuo at 60°C to give the 4-
diamino-5-methyl-6-[(3,4,5-trimethoxyanilino)methyl]quinazoline D_x0002_glucuronate as a yellow powder with no definite melting point.
Potent lipophilic inhibitor of dihydrofolate reductase (DHFR) that displays antineoplastic and antiprotozoal properties. Inhibits protozoal, bacterial and human DHFR with IC 50 values of 1.4, 6.1 and 3.2 nM respectively. Affects G 1 /S phase cell cycle progression.