ChEBI: Palytoxin is a polyol marine coelenterate toxin composed of substituted N-3-hydroxypropyl-trans-3-amidoacrylamides and produced by species of Palythoa and Zoanthus soft corals (collectively called zoantharians), either as a defence mechanism or to assist them in capturing prey. An ionophore that forms cation channels through Na+/K+-ATPase, it is a potent vasoconstrictor useful in evaluation of anti-angina agents. It is considered to be one of the most poisonous non-protein substances known, second only to maitotoxin in terms of toxicity in mice. It has a role as a toxin.
A deadly poison by intraperitoneal, parenteral, subcutaneous, intravenous, intramuscular, and intratracheal routes. A skin and severe eye irritant. When heated to decomposition it emits toxic fumes of NOx.
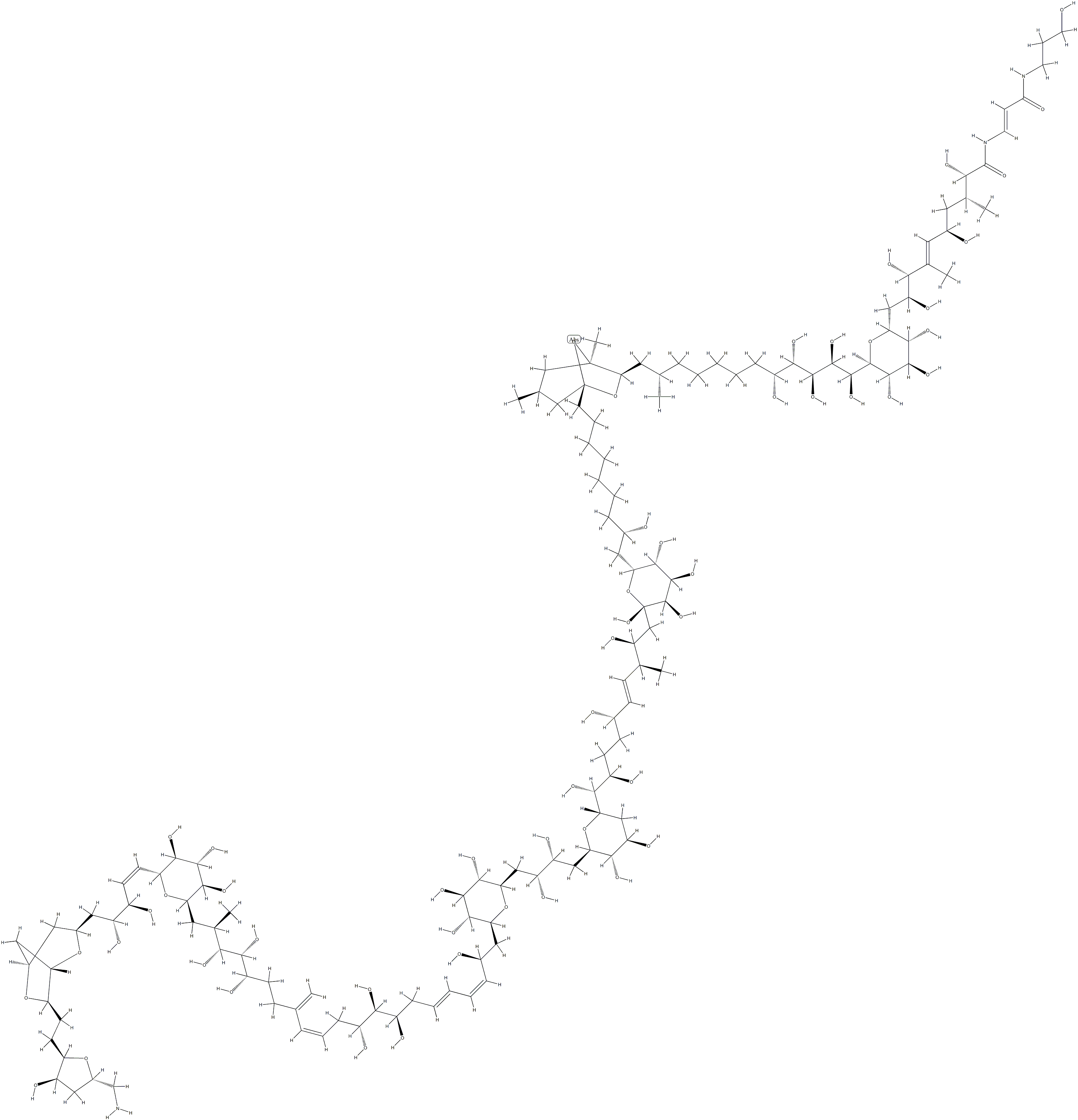
This structurally complex neurotoxin (FW = 2680.17 g/mol; CAS 11077-
03-5; Abbreviation: PLTX), from the Pacific soft coral Palythoa toxica,
binds slowly, but with extreme affinity, to Na+/K+-exchanging ATPase,
converting the latter into an open channel, allowing unhindered passage of
potassium and sodium ions. Less than 1 pM palytoxin is required for rapid
potassium ion outflow from erythrocytes, thereby dissipating its
transmembrane electric potential. Palytoxin is lethal in mice,
exhibiting an impressive LD50 of 15 ng/kg. The pore-forming action of
palytoxin is not restricted to Na+/K+-ATPase, but is also observed with the
colonic H+/K+-ATPase. This observation raises the likelihood that the
toxin reacts at functionally and/or structurally similar manner with these
pumps. PLTX brings about depolarization of the cellular membrane,
disrupting intracellular calcium concentration ([Ca2+]i) and leading to
smooth and cardiac muscle contraction, cytoskeletal dysregulation, and
neurotransmitter release. Second only to maitotoxin as the most toxic
natural product known, palytoxin evokes prompt onset of severe agina and
asthma-like airway effects, followed quickly by tachycardia and death,
often within minutes. SKF-96365 and Gd3+ are widely used to block store-
operated and stretch-activated Ca2+ channels, respectively. SKF-96365 fails
to affect the long-lasting phase elicited by PLTX, excluding the activation
of the store-operated channels during this phase. In contrast, Gd3+
abolishes the long-lasting phase of the [Ca2+]i increase, suggesting a
possible role for stretch-activated channels in PLTX action. While Gd3+ is
also known to inhibit voltage-dependent Ca2+ conductance in neural cells,
voltage-dependent Ca2+ blockade seems unlikely in muscle cells. Even at a
level (100 μM) commonly used to investigate stretch-activated channels
activity, Gd3+ does not affect the transient phase as Verapamil and La3+/Cd2+
does. In addition to the selective inhibitory action of Gd3+ on the long-
lasting phase, Gd3+ also significantly reduces, albeit incompletely, the toxic
effects of PLTX on skeletal muscle cells, suggesting a role for the stretch-
activated channels in the chain of events culminating in death of cultured
muscle cells. Palytoxin also binds to erythrocyte Band-3 protein (B3 or
AE1), altering the anionic flux and seriously compromising not only CO2
(bicarbonate) transport, thereby also affecting hemoglobin
oxygenation/deoxygenation. The stereocontrolled synthesis of
palytoxin was heralded as one of the most complicated syntheses ever
undertaken. (See Maitotoxin)