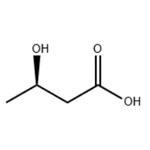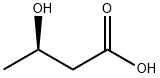
(R)-3-Hydroxybutyric acid
- Product Name(R)-3-Hydroxybutyric acid
- CAS625-72-9
- MFC4H8O3
- MW104.1
- EINECS210-909-6
- MOL File625-72-9.mol
Chemical Properties
| Melting point | 49-50 °C(lit.) |
| alpha | -25 º (C=6% IN H2O) |
| Boiling point | 90-92 °C(Press: 0.08 Torr) |
| Density | 1.195±0.06 g/cm3(Predicted) |
| Flash point | 112 °C |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Oil to Semi-Solid |
| pka | 4.36±0.10(Predicted) |
| color | Colourless to Pale Yellow |
| optical activity | [α]20/D 25±1°, c = 6% in H2O |
| BRN | 1720568 |
| Cosmetics Ingredients Functions | PLASTICISER HUMECTANT ANTI-SEBUM |
| InChI | InChI=1S/C4H8O3/c1-3(5)2-4(6)7/h3,5H,2H2,1H3,(H,6,7)/t3-/m1/s1 |
| InChIKey | WHBMMWSBFZVSSR-GSVOUGTGSA-N |
| SMILES | C(O)(=O)C[C@H](O)C |
| CAS DataBase Reference | 625-72-9(CAS DataBase Reference) |
| EPA Substance Registry System | Butanoic acid, 3-hydroxy-, (3R)- (625-72-9) |
Safety Information
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| F | 3-10 |