(1-Methyl-4-piperidinyl)[3-[2-(3-chlorophenyl)ethyl]pyridinyl]methanone hydrochloride is prepared by the reaction of 4-chloro-1-methylpiperidine and 3-(3-chlorophenethyl)picolinonitrile. The specific synthesis steps are as follows:
Add magnesium (3.6g) and a small amount of iodine into the reaction flask under nitrogen protection,Add re-distilled THF (20ml), 1,2-dibromoethane (1ml), 4-chloro-N-methylpiperidine (1ml), and stir at 75°C; after the liquid surface of the system is foamed, add 4- Chloro-N-methylpiperidine (20.4ml) was dissolved in THF (240ml) and slowly added dropwise. After the dropwise addition, it was reacted for 1h and cooled to room temperature for later use. Compound V (26.0g, 107mmol) was dissolved in THF (250ml) under nitrogen protection;Add the prepared Grignard reagent dropwise to the reaction flask at room temperature to control the temperature of the system; after the dropwise addition, control the temperature of the system at 40-50°C and continue the reaction for 1 hour. After the reaction is completed, adjust the pH of the reaction solution to below 2 with a 2mol/L HCl solution, and continue the reaction at room temperature for 1 hour; after the reaction is completed, THF is distilled off under reduced pressure, and then the pH value of the solution is adjusted with a 50% NaOH solution. Adjust to 3-4, crystallize in an ice-water bath, filter and wash, and dry the solid to obtain a pale yellow powder ((1-Methyl-4-piperidinyl)[3-[2-(3-chlorophenyl)ethyl]pyridinyl]methanone hydrochloride, 33.8 g, 83.3%).
![(1-Methyl-4-piperidinyl)[3-[2-(3-chlorophenyl)ethyl]pyridinyl]methanone hydrochloride synthesis (1-Methyl-4-piperidinyl)[3-[2-(3-chlorophenyl)ethyl]pyridinyl]methanone hydrochloride synthesis](/NewsImg/2024-01-08/6384033139696020939320129.png)
![(1-Methyl-4-piperidinyl)[3-[2-(3-chlorophenyl)ethyl]pyridinyl]methanone hydrochloride Structure](/CAS/GIF/119770-60-4.gif)
![(1-Methyl-4-piperidinyl)[3-[2-(3-chlorophenyl)ethyl]pyridinyl]methanone hydrochloride synthesis (1-Methyl-4-piperidinyl)[3-[2-(3-chlorophenyl)ethyl]pyridinyl]methanone hydrochloride synthesis](/NewsImg/2024-01-08/6384033139696020939320129.png)
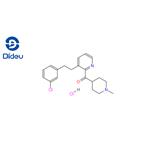
![(1-Methyl-4-piperidinyl)[3-[2-(3-chlorophenyl)ethyl]pyridinyl]methanone hydrochloride pictures](/ProductImageEN/2022-10/Small/95f67a1d-56bf-4b38-9785-848a18e6ca36.png)