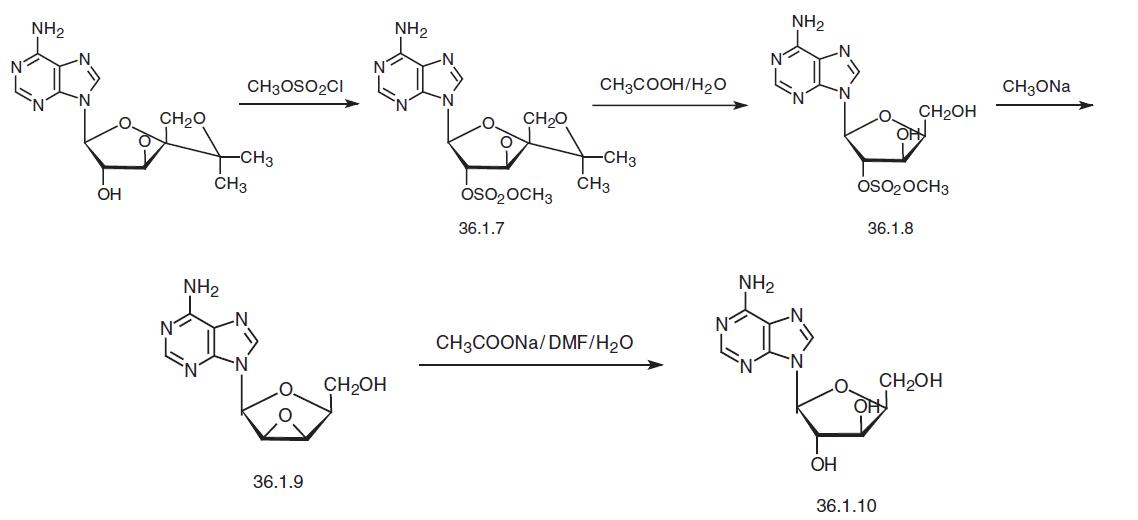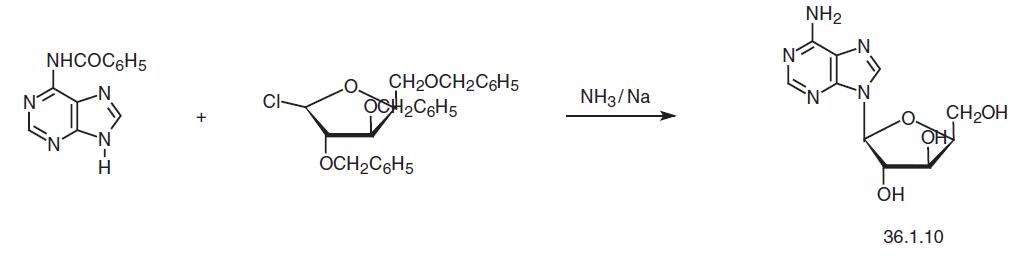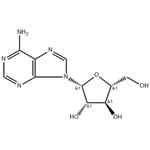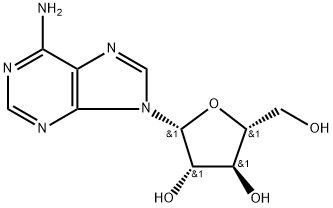
Vidarabine
- Product NameVidarabine
- CAS5536-17-4
- MFC10H13N5O4
- MW267.25
- EINECS226-893-9
- MOL File5536-17-4.mol
Chemical Properties
| Melting point | 260-265 °C (dec.) |
| alpha | D27 -5° (c = 0.25) |
| Boiling point | 410.43°C (rough estimate) |
| Density | 1.3382 (rough estimate) |
| refractive index | 1.7610 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly, Heated) |
| form | Powder |
| pka | pKa 3.55(H2O t=20 I=0.1 (KCl)) (Uncertain);11.4 (Uncertain) |
| color | White to Off-white |
| Water Solubility | Soluble in DMF (10 mg/ml), 0.5 M HCl (50 mg/ml), DMSO (53 mg/ml at 25°C), ethanol (<1 mg/ml at 25°C), and water (3 mg/ml at 25°C). |
| Merck | 13,10039 |
| BRN | 624881 |
| InChI | 1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7+,10-/m1/s1 |
| InChIKey | OIRDTQYFTABQOQ-UHTZMRCNSA-N |
| SMILES | Nc1ncnc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O |
| LogP | -0.755 (est) |
| CAS DataBase Reference | 5536-17-4(CAS DataBase Reference) |
Safety Information
| Hazard Codes | Xn,Xi |
| Risk Statements | 63-36/37/38 |
| Safety Statements | 36/37-36-26 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| RTECS | AU6200000 |
| F | 10-23 |
| TSCA | TSCA listed |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| HS Code | 29349990 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Repr. 2 |
| Hazardous Substances Data | 5536-17-4(Hazardous Substances Data) |
| Toxicity | LD50 in mice (mg/kg): 4677 i.p.; >7950 orally (Kurtz) |