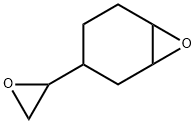
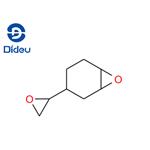
Vinyl cyclohexene dioxide is a colorless liquid
colourless liquid. Combustible.
Vinylcyclohexene dioxide (VCD) is used to study toxicity as an ovotoxin,and it's also used to understand its mechanisms of toxicity towards ovarian follicles and effects on epithelial differentiation.
Vinylcyclohexene dioxide (VCD) may be used to study and understand its effect on ovarian follicles and effects on epithelial differentiation.
As a chemical intermediate and as a
reactive diluent for diepoxides and epoxy
resins.
VCHD is manufactured by epoxidation of 4-vinylcyclohexene
with peroxyacetic acid .
ChEBI: The diepoxide of 4-vinylcyclohexene.
Clear colorless liquid. Sets to glass at -67°F. Faint olefinic odor.
Water soluble. Hydrolyzes slowly in water.
4-VINYLCYCLOHEXENE DIOXIDE reacts with active hydrogen compounds (such as alcohols and amines). . Epoxides are highly reactive. They polymerize in the presence of catalysts or when heated. These polymerization reactions can be violent. Compounds in this group react with acids, bases, and oxidizing and reducing agents. They react, possibly violently with water in the presence of acid and other catalysts.
Toxic by ingestion and skin absorption,
strong irritant to skin and tissue. Female and male
reproductive damage. Possible carcinogen.
Vinyl cyclohexene dioxide
(VCD) is an irritant to the skin, eyes, and respiratory
system. It is ovotoxic and carcinogenic
in experimental animals.
Confirmed carcinogen
with experimental carcinogenic and
tumorigenic data. Poison by unspecified
route. Moderately toxic by ingestion and
skin contact. Mildly toxic by inhalation.
Experimental reproductive effects. Mutation
data reported. A severe skin irritant.Combustible when exposed to heat or
flame. To fight fire, use water, foam, dry
chemical. When heated to decomposition it
emits acrid smoke and irritating fumes.
This material is used as a monomer in
the production of epoxy resins for coatings and adhesives;
as a chemical intermediate and as a reactive diluent.
4-Vinyl-1-cyclohexene diepoxide is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity fromstudies in experimental animals.
UN2810 Toxic liquids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
When heated or in contact with catalysts,
epoxides may cause violent polymerization. Epoxides are
incompatible with reducing agents and oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. May react, possibly violently,
with water in the presence of acid and other catalysts.
Reacts with alcohols, amines and other active
hydrogen compounds. Slowly hydrolyzes in water.
Concentrated waste containing
no peroxides: discharge liquid at a controlled rate near
a pilot flame. Concentrated waste containing peroxides:
perforation of a container of the waste from a safe distance
followed by open burning.