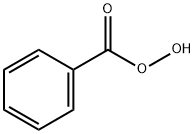
Volatile solid with a pungent odor; sublimes;mp 41–43°C (105.8–109°F); bp 100–105°C(212–221°F) at 15 torr (partially decomposes); slightly soluble in water but mixesreadily with most organic solvents.
Peroxybenzoic acid is used in organic analyses to measure the degree of unsaturation,and in making epoxides.
Perbenzoic acid is used to convert ethylenic compounds into oxides; in analysis of unsatd compounds, to determine the number of double bonds.
The toxicity of peroxybenzoic acid is verylow on animals. In humans it is almostnontoxic. Peroxybenzoic acid caused skintumor in mice on prolonged contact (NIOSH1986). Its tumorigenic action was lower thanthat of peroxyacetic acid. Its carcinogenicityin humans is unknown.
The presence of the peroxide functional
group renders its strong oxidizing properties similar to those of other peroxy compounds. However, this compound is not
hazardous like peroxyacetic or peroxyformic
acids. It is not shock sensitive. Violent
decomposition may occur only at high temperatures and/or in conjunction with certain organic contaminants that are easily
oxidizable. Although there is no report
of its explosion, general safety measures
for handling peroxy compounds should be
followed.
Moderately irritating to skin, eyes, and mucous membranes by ingestion and inhalation. Questionable carcinogen with experimental tumorigenic data by skin contact. A dangerous fire hazard when exposed to heat, flame, or reducing materials. A powerful oxid
Crystallise the peracid from *benzene or pet ether. It sublimes readily and is steam volatile. It is soluble in CHCl3, CCl4 and Et2O. [Braun Org Synth Coll Vol I 431 1941.] EXPLOSIVE.
The initial threshold screening level (ITSL) for peroxybenzoic acid is being set at 0.1 μg/m3 with annual averaging.