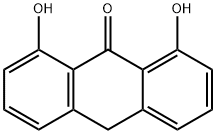
ANTHRALIN
- Product NameANTHRALIN
- CAS1143-38-0
- MFC14H10O3
- MW226.23
- EINECS214-538-0
- MOL File1143-38-0.mol
Chemical Properties
| Melting point | 178-181 °C (lit.) |
| Boiling point | 464.1±45.0 °C(Predicted) |
| Density | 1.419±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, soluble in methylene chloride, sparingly soluble in acetone, slightly soluble in ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides. |
| form | Solid |
| pka | 7.16±0.20(Predicted) |
| color | Yellow to Orange to Dark Yellow |
| Water Solubility | Insoluble in water. Soluble in acetic acid, chloroform (20 mg/mL), acetone, benzene, solutions of alkali hydroxides, fixed oil. Slightly soluble in 95% ethanol, alcohol, ether, and glacial acetic acid. |
| Merck | 14,684 |
| BRN | 2054360 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChI | 1S/C14H10O3/c15-10-5-1-3-8-7-9-4-2-6-11(16)13(9)14(17)12(8)10/h1-6,15-16H,7H2 |
| InChIKey | NUZWLKWWNNJHPT-UHFFFAOYSA-N |
| SMILES | Oc1cccc2Cc3cccc(O)c3C(=O)c12 |
| CAS DataBase Reference | 1143-38-0(CAS DataBase Reference) |
| IARC | 3 (Vol. 13; Sup 7) 1987 |
| EPA Substance Registry System | Dithranol (1143-38-0) |
Safety Information
| Hazard Codes | Xi,T |
| Risk Statements | 36/37/38-45 |
| Safety Statements | 26-53-45 |
| WGK Germany | 3 |
| RTECS | CB8927000 |
| HS Code | 29144000 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |


