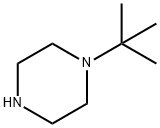
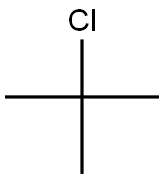
General procedure for the synthesis of N-tert-butylpiperazine from chlorinated tert-butane and compound (CAS:7542-23-6): 20 mmol of compound (1) and 150 mL of tetrahydrofuran were added to a 250 mL round bottom flask. A 50 mL acetonitrile solution of 20 mmol of tert-butyl chloride was slowly added dropwise with stirring at room temperature. The dropwise addition process was completed in about 2 hours. Stirring of the reaction mixture was continued for 2 hours. It was then heated to reflux and stirred for 5 h. After completion of the reaction, the reaction was cooled to room temperature, filtered, and the solid was washed with 10 mL of tetrahydrofuran and dried to give mono-substituted piperazinic acid dihydrochloride (5) (R=tert-butyl). The compound (5) was transferred to a 250 mL round bottom flask and 200 mL of tetrahydrofuran and an appropriate amount of base was added. The reaction was heated to reflux for 4 hours under stirring. After completion of the reaction, it was cooled to room temperature, filtered, and the filtrate was evaporated by solvent to remove the low-boiling components, and the product was collected by distillation to afford the light yellow oily liquid N-tert-butylpiperazine (6) (R=tert-butyl) in 92% yield (based on the amount of tert-butyl chloride fed).