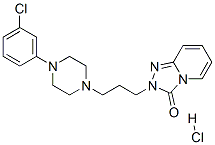
Trazodone Hydrochloride belongs to the hydrochloride salt form of trazodone with antidepressant and sedative activities, which is primarily used in the treatment of major depression. It serves as a serotonin uptake inhibitor that is effective for patients suffering from major depressive disorders and other subsets of depressive disorders, which is especially helpful for geriatric patients with severe agitation and certain depressive disorders associated with insomnia and anxiety since it has proved that trazodone also has anti-anxiety (anxiolytic) and sleep-inducing (hypnotic) effects. Besides, it is often prescribed as a sedative that is used for the treatment of panic attacks, aggressive behavior, agoraphobia, and cocaine withdrawal in combination with other drugs. Trazodone Hydrochloride is an oral drug that functions by affecting the balance of chemicals (neurotransmitters) within the brain that nerves use to communicate with (stimulate) each other.
https://en.wikipedia.org/wiki/Trazodone
http://www.medicinenet.com/trazodone/article.htm
https://www.drugbank.ca/drugs/DB00656
http://bodyandhealth.canada.com/drug/getdrug/ratio-trazodone
https://pubchem.ncbi.nlm.nih.gov/compound/62935#section=Top
Trittico,Angelini,Italy,1972
A serotonin uptake inhibitor
Monoamine reuptake inhibitor; antidepressant.
ChEBI: A hydrochloride salt prepared from equimolar amounts of trazodone and hydrogen chloride.
In an initial step, 2-chloropyridine is reacted with semicarbazide to give s_x0002_triazolo-[4,3-a]-pyridine-3-one.
To a boiling solution of 6.7 grams s-triazolo-[4,3-a]-pyridine-3-one in 80 ml
dioxane, there is added 2.4 grams 50% NaH. The mixture is refluxed during 1
hour under stirring, then 13.5 grams 1-(3-chloropropyl)-4-m�chlorophenylpiperazine is added. The mixture is refluxed under stirring for 20
hours, cooled, diluted with an equal volume of ether, the sodium chloride
filtered out, and ethereal HCl added. The solid which precipitates is filtered out and crystallized from 95% alcohol. Yield is 13.5 grams, MP 223°C.
The following is an alternative method of preparation: 1 gram 2-(γ-
chloropropyl)-s-triazolo-[4,3-a]-pyridine-3-one and 5 ml saturated ammonia
alcoholic solution are heated for 5 hours in a closed tube at 100°C. The
contents of the tube are cooled, the ammonium chloride filtered out and the
solvent is removed. There remains a residue of 0.9 grams 2-(γ-aminopropyl)-
s-triazolo-[4,3-a]-pyridine-3-one.
This residue is dissolved in isopropyl alcohol and 1 gram N-bis-chloroethyl�aniline is added to it. The mixture is refluxed for 3 hours. The solvent is
removed at a reduced pressure, the residue is treated with 50% potassium
carbonate, and extracted with ether. By treating with ethereal hydrochloric
acid, 2-N'-m-chlorophenylpiperazino-propyl-s-triazole[4,3-a]pyridine-3-one
hydrochloride is precipitated; MP 223°C.
Trazodone is a antidepressant drug sold under trade names such as Desyrel and Oleptro for treatment of anxiety disorder, depression, and insomnia. Trazadone, a serotonin antagonist and reuptake inhibitor (SARI), also has anti-anxiety and hypnotic effects. This certified Snap-N-Spike solution is suitable for use in clinical toxicology, forensic analysis, or urine drug testing methods by LC-MS/MS or GC/MS.
Antidepressant that potentiates the activity of serotonin uptake blockers and has full 5-HT2C serotonin receptor agonist activity. It is metabolized to the 5-HT1 serotonin receptor agonist 1-(3-Chlorophenyl)piperazine.
Tricyclic-related antidepressant
Potentially hazardous interactions with other drugs
Alcohol: increased sedative effects.
Antidepressants: avoid concomitant use with
MAOIs and moclobemide.
Antiepileptics: antagonism of anticonvulsant effect;
concentration reduced by carbamazepine.
Antimalarials: manufacturer advises avoid
concomitant use with artemether and lumefantrine
and piperaquine with artenimol.
Antivirals: concentration increased by ritonavir;
increased risk of ventricular arrhythmias with
saquinavir - avoid; concentration possibly increased
by telaprevir.
Trazodone is hepatically metabolised via the cytochrome P450 isoenzyme CYP3A4 by n-oxidation and hydroxylation. The metabolite m-chlorophenylpiperazine is active. Trazodone is excreted in the urine almost entirely in the form of its metabolites.