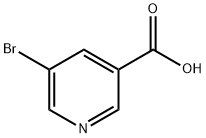
5-Bromopyridine-3-carboxylic acid (5-bromonicotinic acid) was used in the synthesis of 3-guanidinomethyl-5-iodopyridine.
5-Bromonicotinic acid could used as starting material to synthesize 3-guanidinomethyl-5-iodopyridine. It is also used to prepare four novel multifunctional coordination compounds with different lanthanide(III) ions (Dy, Tb, Yb, and Nd). These materials possess different structures and dimensionalities and show interesting magnetic and luminescence properties, as well as a complete absence of cytotoxicity both in cancer and non-cancer Caco-2 cells[1-2].
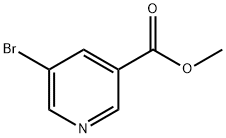
General procedure for the synthesis of 5-bromonicotinic acid from methyl 5-bromonicotinate: to a solution of methyl 5-bromonicotinate (500 mg, 2.3 mmol) in tetrahydrofuran (THF, 5 mL) was added an aqueous 1 N sodium hydroxide solution (5 mL, 2.3 mmol). The reaction mixture was stirred at room temperature for 10 min. Subsequently, the reaction solution was acidified to pH 6 with acetic acid and then extracted three times with ethyl acetate. The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 5-bromonicotinic acid as a white solid (153 mg, 33% yield).
The acid is recrystallised from H2O and then from EtOH using charcoal. The amide has m 219-219.5o (from aqueous EtOH), and the methyl ester, prepared by addition of ethereal diazomethane, can be purified by sublimation in a vacuum and has m 98-99o. The acid chloride also can be sublimed in vacuo and has m 74-75o and gives the methyl ester in MeOH. [Graf J Prakt Chem 138 244 1933, Bachman & Micucci J Am Chem Soc 70 2381 1948, Garcia et al. J Am Chem Soc 82 4430 1960, Misic-Vokovic et al. J Chem Soc 34 1978, Beilstein 22/2 V 181.]
[1] Vaidyanathan G, et al. Iodopyridine-for-iodobenzene substitution for use with low molecular weight radiopharmaceuticals: application to m-iodobenzylguanidine. Bioconjugate Chemistry, 1998; 9: 758–764.
[2] Ruiz C, et al. Multifunctional coordination compounds based on lanthanide ions and 5-bromonicotinic acid: magnetic, luminescence and anti-cancer properties. CrystEngComm, 2019; 21: 3881-3890.