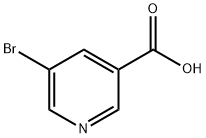
5-Bromonicotinic acid: a nicotinic acid containing bromine
5-Bromonicotinic acid (5BNA) is one of the derivatives of nicotinic acid containing the halogen bromine in it. It has a molecular formula of C6H4BrNO2 and a mass of 202.01 u and has been selected for quantum chemical investigation. A computational technique emphasised by Density Functional Theory (DFT) is used to determine the physiochemical properties of 5-Bromonicotinic acid. A comprehensive study of the literature revealed that the computational assessment of the molecule in question has not yet been done, so we studied it using DFT calculations.[1]

Structure analysis and Non-linear optics of 5-Bromonicotinic acid
The 5-Bromonicotinic acid is computationally optimised as shown and we give the bond characteristics of its gaseous form as well as in different solvents. The XRD results of the 5-Bromonicotinic acid show that it has a triclinic structure with intercepts of 8.2576 Å, 11.0316 Å and 17.648 Å and has angles 93.9010°, 95.644° and 91.486°. There is one Br–C bond, two O–C bonds, two N–C bonds, two O–H bonds, three C–H bonds, and five C–C bonds in this compound. The highest bond length is observed at O3–H14 and Br1–C7. The bond between O3–H14 is an intermolecular interaction, especially a partial link such as a hydrogen bond. In Br1–C7, the longer bond length with the carbon atom results from the presence of bromine, which is heavy in size and has a decreased electronegativity.
Non-linear optics is the evaluation of phenomena that develop as a result of light's influence altering a material's optical characteristics. It focuses on the theoretical underpinnings regulating the tensor properties of a molecule's polarisation capability. We demonstrates the calculated NLO parameters such as dipole moment (μ), polarizability (α) and hyperpolarizability (β) of 5-Bromonicotinic acid with the various solvents. The calculated values of μ,α and β of 5BNA in the gas phase are 0.68666D, 16.54292 × 10−24 esu and 2499.598978 × 10−33 esu. 5-Bromonicotinic acid with various solvents could serve as a suitable NLO compound and its large α, β and no null values of μ suggest that it belongs to an essential group of compounds in pharmaceutical science. Moreover, in comparison with nicotinic acid, 5-Bromonicotinic acid shows high enhancement as the bromine atom in the 5BNA acts as an electron-withdrawing group. The prediction of μ plays a significant subject related to the stability of the molecule. These obtained findings are made to compare with the urea as the model and it is noticed that the 5BNA in gas and with solvents have larger values of α and β. As a result, it is possible 5-Bromonicotinic acid will be useful for research on optical properties that are nonlinear over the forthcoming years.[2]
Electronic analysis
The UV–Visible spectrum of 5-bromonicotinic acid in gas, water, chloroform, diethyl ether, and acetonitrile has been determined using the TD-DFT technique and illustrated. The absorption energy, wavelength, energy band gap and Molecular orbitals contributions are tabulated. The impact of solvents is investigated using the IEFPCM model. Between 255 and 295 nm, a broad absorbance spectrum of 5BNA in the gas phase was found. Diethyl ether has the greatest influence on the UV transition, which is followed by chloroform, acetonitrile and finally water. The highest wavelengths for gas, diethyl ether, chloroform, acetonitrile and water have been found to be 299.17 nm, 294.44 nm, 294.25 nm and 291.71 nm and 291.45 nm respectively. With the addition of solvents, there is an increase in energy as well as in the band gap of 5-bromonicotinic acid. The electronic transitions π to π* will shift to longer wavelengths whereas, n to π* and n to σ* will shift towards the lower wavelengths when the polarity of the solvent increases. According to the absorption study, there was a slight hypochromic shift in the absorption spectra when the solvents were incorporated. The shifted absorption spectra show that solvents are involved in causing the intramolecular interaction in the molecule. Furthermore, the spectral shifts were caused by the HOMO and LUMO charge cloud altering with the lowest energy. Consequently, the calculated wavelengths 299.17 nm, 291.45 nm, 294.25 nm, 294.44 nm and 291.71 nm for the 5-bromonicotinic acid in gas as well as with the solvents water, chloroform, diethyl ether and acetonitrile show the n to π* transition.
DFT Studies on 5-Bromonicotinatic Acid
The DFT approach is employed to theoretically characterize the structural and electronic characteristics of 5-Bromonicotinatic acid by using different organic solvents. The various reactive and fingerprint zones present in the compound are picturised using the ESP map, Fukui analysis and Hirshfeld surface. μ, α and β of 5BNA exhibit a shift when they switch between the gas to the solution phases. Through the NBO analysis, the highest stabilisation energy of 47.29 kcal/mol is found for the transition between the LP O2→ π* O3–C10. In 5-bromonicotinic acid, the majority of transitions occur between the π and π*. The molecular orbital energy difference of 5BNA with the diethyl ether has the highest energy difference of 5.46522eV compared with other solvents and gas phase of 5-bromonicotinic acid. The highest values of the D index i.e., <1 and the H index indicate the presence of charge transfer within the molecule.
The highest values of ρ and ∇2ρ by AIM analysis at the N4–C9, C8–C9, C7–C8, C6–C7 and C5–C6, indicate that the 5-bromonicotinic acid in both gas phase as well as with solvent show maximum electron density and covalent nature. By ELF and LOL studies, the electron dense region is found at the circumference of the aromatic ring and the electron-deficit zone is found at the carbon atoms. By the UV–Vis spectra, the maximum absorption wavelength is observed at 299.17 nm for gas and 294.44 nm for diethyl ether. The various molecular vibrations present in the compound are determined and documented using vibrational spectroscopy (IR and Raman) and their wavenumbers are correlated with the experimental values. 5-bromonicotinic acid 's drug-likeness and toxicity effects show that compound 5BNA can be suggested as a drug. The proteins 2A4O, 6CWD and 2OC8 associated with Hepatitis A, Hepatitis B and Hepatitis C are docked with the ligand 5BNA and has the binding energy of −4.7 kcal/mol, −5.3 kcal/mol and −5.2 kcal/mol.
Derivatization method with 5-bromonicotinic acid N-hydroxysuccinimide
Scientists have developed a novel method that effectively identifies the N-terminal product ions produced in the tandem mass spectrometry (MS/MS) analysis of peptides done in conjunction with the specific derivatization of the N-terminal amino group using 5-bromonicotinic acid N-hydroxysuccinimide ester (BrNA-NHS). Electrospray ionization with low-energy collision-induced dissociation (CID) MS/MS clearly differentiated the N-terminal product ions labeled with the 5-bromonicotinyl group from other ions, on the basis of the appearance of CID peaks with a doublet pattern characteristically separated by 2 mass units produced by the equal natural abundances of 79Br and 81Br. The tracing of a series of these bromine-containing product ions allows the easy amino acid sequencing of peptides. Using Gln-Arg-Leu-Gln-Ser-Asn-Gln-Leu-Lys as the test peptide, we found that within 30 minutes at pH 6.5 and 37 degrees C its alpha-amino group was completely acylated with 5-bromonicotinic acid N-hydroxysuccinimide ester.[3]
References
[1]Behrman Edward, Stanier R. Oxidation of halogenated nicotinic acids. J. Biol. Chem. 1957;228:947–953.
[2]R S, Mahalakshmi S, Kumaran S, Kadaikunnan S, Abbas G, Muthu S. Structural, electronic, intermolecular interaction, reactivity, vibrational spectroscopy, charge transfer, Hirshfeld surface analysis, pharmacological and hydropathy plot on 5-Bromo nicotinic acid - Antiviral study (Hepatitis A, B, and C). Heliyon. 2023 Sep 7;9(9):e19965.
[3]Miyagi M, Nakao M, Nakazawa T, Kato I, Tsunasawa S. A novel derivatization method with 5-bromonicotinic acid N-hydroxysuccinimide for determination of the amino acid sequences of peptides. Rapid Commun Mass Spectrom. 1998;12(10):603-8.
See also
Lastest Price from 5-Bromonicotinic acid manufacturers

US $10.00/KG2025-04-21
- CAS:
- 20826-04-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $10.00/kg2025-04-21
- CAS:
- 20826-04-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 ton