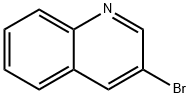
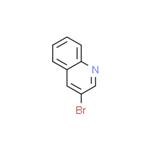
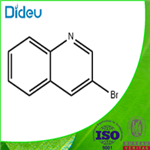
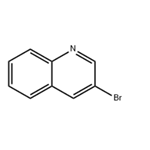
There are seven position isomers with the main properties are as follows:
Name
Melting point(℃)
Boiling point(℃)
Solubility
2-bromoquinoline
48~49
It is soluble in diethyl ether, chloroform and benzene
3-bromoquinoline
12~15
274~276,
95(66.66Pa)
4-bromoquinoline
29~30
270(decomposition)
Easily soluble in dilute acid
5-bromoquinoline
52 (needle crystal)
280
7-bromoquinoline
52 (needle crystal)
290
8-bromoquinoline
80
165~166
(2399.79Pa)
Application and synthesis method
3-Bromoquinoline can have action with mixed acid to generate 3-bromo-5-nitroquinoline, followed by heating with potassium permanganate to be oxidized to 5-bromo-2, 3-pyridine dicarboxylic acid.
6-bromo-quinoline can be heated together nitric acid to generate 6-bromo-8-nitro-quinoline, followed by reaction with potassium permanganate to be oxidized into 2, 3-pyridinedicarboxylic acid.
2-bromo-quinoline can be manufactured through the reaction between 2-hydroxyquinoline and phosphorus pentabromide
3-bromo-quinoline can be obtained through heating the quinoline perbromide at 180 ° C.
4-bromoquinoline can be obtained through either the heating reaction between 4-hydroxyquinoline and phosphorus pentabromide or by the diazotization reaction of 4-aminoquinoline.
5-bromo-quinoline can be obtained from the heating reaction between m-bromo aniline, glycerol, m-bromonitrobenzene and concentrated sulfuric acid, or through the diazotization reaction of 5-amino-quinoline.
6-bromo-quinoline can be obtained through the heating reaction between p-bromoaniline, glycerol, concentrated sulfuric acid and p-bromo-nitrobenzene.
7-bromoquinoline can be obtained through the diazotization of 7-aminoquinoline.
8-bromo-quinoline can be obtained through the heating of o-bromo aniline, glycerol, concentrated sulfuric acid and o-bromo-nitrobenzene in the heating system.
Purposes: as organic synthesis reagents.
For pharmaceuticals
Medicine, pesticide intermediates
colorless to light yellow liquid
3-Bromoquinoline undergoes bromine-magnesium exchange reaction with lithium tributylmagnesate in toluene at -10°C, which is quenched by various electrophiles to yield functionalized quinolines.
3-Bromoquinoline undergoes bromine-magnesium exchange reaction with lithium tributylmagnesate in toluene at -10°C, which is quenched by various electrophiles to yield functionalized quinolines.
Flammability and Explosibility
Not classified
[1] Patent: US3956301, 1976, A
[2] Patent: US5266700, 1993, A