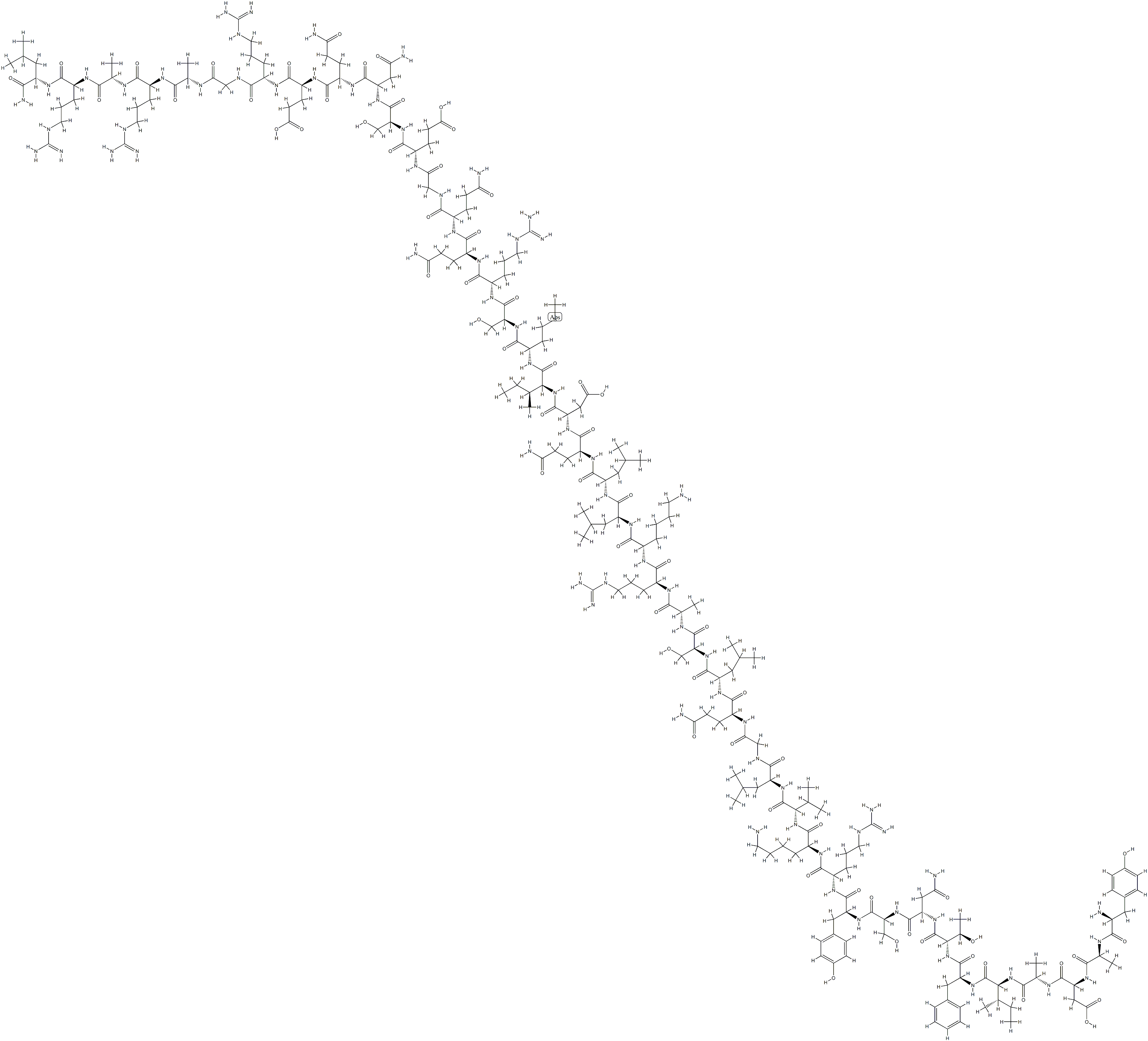GHRH(1–29) is the bioactive core of human GHRH. The N-terminal tyrosine residue with
selected aromatic rings is important for the high bioactivity in human and nonrodent mammalian GHRH. The amino acid sequence of GHRH shows higher identities between the human, porcine, bovine, and caprine species, but the rat and mouse are exceptions. The
sequence of the C-terminus is highly variable among species while the N-terminus is more conserved. The N-terminal region (1–27) of GHRH is well conserved in nonmammalian vertebrates. Zebrafish GHRH
(1–27) shows 74.1%, 81.5%, and 81.5% similarity to the
Xenopus tropicalis, chicken, and human counterparts,
respectively. Mr 12,447 (GHRH(1-44), Mr 5,039), pI 10.3 (GHRH(1-
44), pI 11.5). Soluble in acidic aqueous solution (e.g., 1%
acetic acid). Lyophilized GHRH is stable at room temperature for 2months, and recommended storage is below
-18°C with desiccation.
Gene, mRNA, and precursor
The human GHRH gene, GHRH, location 20q11.2, consists of five exons. GHRH mRNA has 459 bases that
encode a signal peptide of 24 aa residues, a mature protein of 44 aa residues, and a C-peptide of 31 aa residues
with unknown function. In nonmammalian
vertebrates, the GHRH-like peptide and PACAP were
first believed to be encoded by the same gene, but later
actual GHRH and PACAP were found to be encoded
by two distinct genes.
The human GHRH gene, GHRH, location 20q11.2, consists of five exons. GHRH mRNA has 459 bases that
encode a signal peptide of 24 aa residues, a mature protein of 44 aa residues, and a C-peptide of 31 aa residues
with unknown function. In nonmammalian
vertebrates, the GHRH-like peptide and PACAP were
first believed to be encoded by the same gene, but later
actual GHRH and PACAP were found to be encoded
by two distinct genes.
The synthesis and release of GHRH are regulated by
sex hormones, aging, the negative feedback effect of
GH, and diverse pathological conditions. Gsh-1 has been
considered a transcriptional factor of Ghrh expression in
the rat hypothalamus. GHRH synthesis is inhibited by
somatostatin (SS). The expression levels of the SS receptor, sst2A, in GHRH neurons are higher in female mice
than male mice. The production of hypothalamic GHRH
is decreased by aging. It is also negatively regulated by
the feedback of GH, whereas ghrelin stimulates GHRH
release.
GHRH-R belongs to the GPCR B II subclass, highly
selective for GHRH. The GHRH-R of most mammals
consists of 423 aa residues. The N-terminal extracellular
domain contains a site for N-glycosylation as well as
six cysteine residues and an aspartate residue that are
conserved in this receptor family. The third intracellular
loop and the C-terminal intracellular domain contain several potential phosphorylation sites, which may regulate
signaling and receptor internalization. It is mainly
expressed in the pituitary.
Tesamorelin, sermorelin (GHRH(1–29)-NH2), and
CJC-1295 are agonists. Antagonists comprise the antibodies or peptides to
GHRH-R: JV-1-10, JV-1-36, JV-1-37, JV-1-38, JV-1-39,
JV-1-40, JV-1-41, JV-1-42, JV-1-43, JV-1-62, JV-1-63,
MZ-4-71, MZ-4-169, MZ-4-181, MZ-4-243, MZ-5-78,
MZ-5-156, MZ-5-192, MZ-6-55, [Ac-Tyr1, D-Arg2]
GHRH(1–29)-NH2.
GHRH receptor mRNA is expressed in several organs,
especially in the adrenal, digestive tract, and kidney. The
primary function of GHRH is to stimulate GH synthesis
and release from the anterior pituitary somatotrophs.
GHRH activates cell proliferation, cell differentiation,
and growth of somatotrophs, and is also involved in
the modulation of appetite and feeding behavior, the regulation of sleeping, the control of jejunal motility, and the
increase in leptin levels in modest obesity .
Mutations in the GHRH gene have never been
described. A single base change in the GHRH-R gene in
human somatotropinoma confers hypersensitivity to
GHRH binding. Pit-1 mutation inducing the low gene
expression of GHRH-R can lead to the development of
dwarfism.
GHRH is expressed and secreted from the hypothalamic
neurons of the arcuate nucleus (ARC). GHRH stimulates
the release of growth hormone (GH) in the anterior
pituitary. In 1982, three isoforms of GHRH(1–37, 1–40, 1–44 aa
residues) were initially isolated from human pancreatic
tumors that caused acromegaly, and the latter two were
found in the human hypothalamus. The aa sequence of
GHRH was also identified in various vertebrates from
rodents to fish, including a protochordate. In nonmammalian vertebrates, GHRH-like peptide (pituitary adenylate cyclase-activating polypeptide (PACAP)-related
peptide in mammals) was first isolated like GHRH,
although the GHRH-like peptide had less activity on
GH release. Later, actual GHRH, which was more phylogenetically and structurally similar to mammalian
GHRH and showed GH-releasing activity, was isolated
in nonmammalian vertebrates.
Human growth hormone-releasing factor (Growth Hormone Releasing Factor human) is a hypothalamic polypeptide and stimulates GH production and release by binding to the GHRH Receptor (GHRHR) on cells in the anterior pituitary[1].
Sermorelin, a functional peptide fragment of GHRH
(1–29), has been used in the diagnosis and treatment of
children with idiopathic growth hormone deficiency. Tesamorelin, a stabilized synthetic peptide analog of
GHRH(1–44), received US Food and Drug Administration approval in 2010 for the treatment of lipodystrophy
in HIV patients under highly active antiretroviral therapy, and was investigated for effects on certain cognitive
functions in adults with cognitive impairment as well as
healthy older adults.
Growth Hormone Releasing Hormone Human Synthetic is a single, non-glycosylated, polypeptide chain containing 29 amino acids and having a molecular mass of 3358.9 Dalton.
Corresponds to the amino-terminal segment of the naturally occurring human growth hormone-releasing hormone consisting of 44 amino acid residues.
The GHRH is purified by proprietary chromatographic techniques.
Growth-hormone-releasing hormone (GHRH), also known as growth-hormone-releasing factor (GRF or GHRF) or somatocrinin, is a 44-amino acidpeptide hormoneproduced in the arcuate nucleusof the hypothalamus.
GHRH is released from neurosecretory nerve terminals of these arcuate neurons, and is carried by the hypothalamo-hypophysial portal circulation to the anterior pituitary glandwhere it stimulates growth hormone(GH) secretion. GHRH also stimulates the production of GH. GHRH is released in a pulsatile manner, stimulating similar pulsatile release of GH. In addition, GHRH also promotes slow-wave sleepdirectly.
[1] Fridlyand LE, et al.Growth Hormone-Releasing Hormone in Diabetes.Front Endocrinol (Lausanne). 2016 Oct 10;7:129. eCollection 2016. DOI:
10.3389/fendo.2016.00129