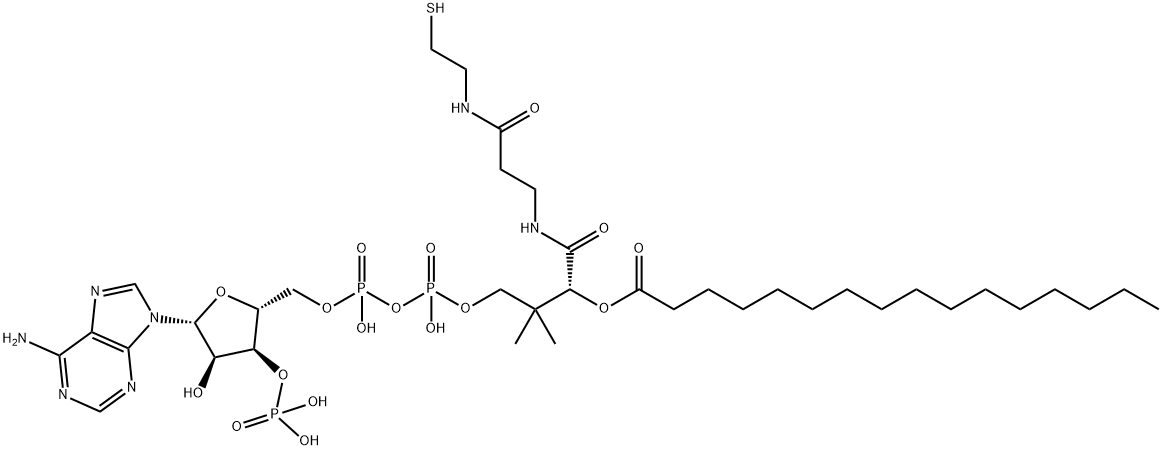
ChEBI: Palmitoyl-CoA is a long-chain fatty acyl-CoA resulting from the formal condensation of the carboxy group of hexadecanoic acid with the thiol group of coenzyme A. It has a role as an Escherichia coli metabolite and a mouse metabolite. It is a long-chain fatty acyl-CoA, a palmitoyl bioconjugate, an 11,12-saturated fatty acyl-CoA and a 3-substituted propionyl-CoA. It is functionally related to a coenzyme A, a hexadecanoic acid and a hexadecanoate. It is a conjugate acid of a palmitoyl-CoA(4-).
Possible impurities are palmitic acid, S-palmitoyl thioglycolic acid and S-palmitoyl glutathione. These are removed by placing ca 200mg in a centrifuge tube and extracting with Me2CO (20mL), followed by two successive extractions with Et2O (15mL) to remove S-palmitoyl thioglycolic acid and palmitic acid. The residue is dissolved in H2O (4 x 4 mL), adjusted to pH 5 and centrifuged to remove insoluble S-palmitoyl glutathione and other insoluble impurities. To the clear supernatant is added 5% HClO4 (6mL) whereby S-palmitoyl CoA precipitates. The precipitate is washed with 0.8% HClO4 (10mL) and finally with Me2CO (3x 5mL) and dried in vacuo. It is stable for at least one year in dry form at 0o in a desiccator (dark). Solutions are stable for several months at -15o. Its solubility in H2O is 4%. The adenine content is used as the basis of purity with max at 260 and 232nm (� 6.4 x 106 and 9.4 x 106 cm2/mol, respectively). Higher absorption at 232nm would indicate other thio ester impurities, e.g. S-palmitoyl glutathione, which absorb highly at this wavelength. Also the phosphate content should be determined, and acid phosphate can be titrated potentiometrically. [Seubert Biochemical Preparations 7 80 1960, Srer et al. Biochim Biophys Acta 33 31 1959, Kornberg & Pricer J Biol Chem 204 329 , 345 1953, Beilstein 26 III/IV 3665.]