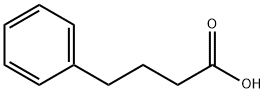
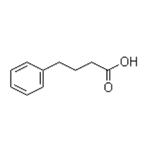
4-Phenylbutyric acid is white to slightly yellowish crystalline powder. It is slightly soluble in chloroform and methanol. Its solubility in water is 5.3 g/l (at 40 °C).
4-Phenylbutyric acid is used as a chemical chaperone involved in protein-folding disorders. It is involved in the synthesis of dyes and an active pharmaceutical ingredient intermediate.
4-phenylbutyric acid is obtained by the reaction of benzene with butyrolactone in the presence of aluminum chloride, followed by neutralization with base.
Synthesis of 4-phenylbutyric acid
4-Phenylbutyric acid (4-PBA) is used in the treatment of urea cycle disorders under the trade name Buphenyl. 4-PBA has been used as a selective inhibitor of endoplasmic reticulum stress (ERS).
4-Phenylbutyric acid is used as organic intermediates. It can also be used as a reactant in the synthesis of:
1-Tetralone using Lewis acid catalyst.
4,N-diphenylbutyramide.
4-Phenylbutyric acid is a monocarboxylic acid the structure of which is that of butyric acid substituted with a phenyl group at C-4. It is a histone deacetylase inhibitor that displays anticancer activity. It inhibits cell proliferation, invasion and migration and induces apopto is in glioma cells. It also inhibits protein isoprenylation, depletes plasma glutamine, increases production of foetal haemoglobin through transcriptional activation of the gamma-globin gene and affects hPPARgamma activation.
Crystallise the acid from pet ether (b 40-60o). [Beilstein 9 IV 1811.]