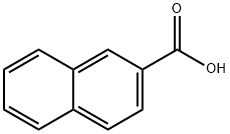
2-Naphthoic acid is an unprotected acid undergoes lithiation, e.g. with s-BuLi, by stereospecific 1,4-addition, providing a facile route to 1,2,2-trisubstituted 1,2-dihydronaphthalenes. It is also used as an intermediate in organic synthesis. The fluorescence spectra and electronic absorption of 2-naphthoic acid was studied.
ChEBI: 2-naphthoic acid is a naphthoic acid that is naphthalene carrying a carboxy group at position 2. It has a role as a xenobiotic metabolite and a mouse metabolite. It is a conjugate acid of a 2-naphthoate.
2-Naphthoic acid (NPA) is a noncompetitive N-methyl-D-aspartate (NMDA) receptor inhibitor. The fluorescence spectra and electronic absorption of 2-naphthoic acid was studied.
Crystallise the acid from EtOH (4mL/g), aqueous 50% EtOH or Me2CO (m 185-186o). Dry it at 100o. The acid chloride has m 52-52o (from *C6H6/pet ether) and b 160-162o/11mm [Hersberg & Carson Org Synth Coll Vol III 629 1955], the amide has m 192o (from EtOH), and the N-Methyl amide has m 109-109.5o (form *C6H6). [Beilstein 9 H 656, 9 III 3174, 9 IV 2402.]