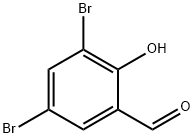
3,5-Dibromosalicylaldehyde
- Product Name3,5-Dibromosalicylaldehyde
- CAS90-59-5
- MFC7H4Br2O2
- MW279.91
- EINECS202-003-4
- MOL File90-59-5.mol
Chemical Properties
| Melting point | 82-83.5 °C (lit.) |
| Boiling point | 261.2±35.0 °C(Predicted) |
| Density | 1.9661 (rough estimate) |
| refractive index | 1.4970 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | methanol: soluble25mg/mL, clear, yellow to brown |
| form | Crystalline Powder |
| pka | 6.08±0.23(Predicted) |
| color | Yellow to yellow-brown |
| Water Solubility | Soluble in methanol 25 mg/mL. Insoluble in water. |
| Sensitive | Air Sensitive |
| Merck | 14,3027 |
| BRN | 1424739 |
| InChI | 1S/C7H4Br2O2/c8-5-1-4(3-10)7(11)6(9)2-5/h1-3,11H |
| InChIKey | JHZOXYGFQMROFJ-UHFFFAOYSA-N |
| SMILES | Oc1c(Br)cc(Br)cc1C=O |
| CAS DataBase Reference | 90-59-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 3,5-Dibromosalicylaldehyde(90-59-5) |
Safety Information
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-37/39-24/25 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 3 |
| RTECS | CU5609000 |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 29130000 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Aquatic Acute 1 Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |


