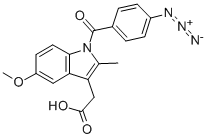
zidometacin
- Product Namezidometacin
- CAS62851-43-8
- MFC19H16N4O4
- MW0
- EINECS263-740-5
- MOL File62851-43-8.mol
Usage And Synthesis
A hot solution of 5.2 g (15.4 mmol) of 1-(p-aminobenzoyl)-5-methoxy-2-
methyl-3-indolylacetic acid in 65 ml of acetic acid is rapidly cooled to 25-30°C
(try to avoid crystallization). This cold solution and a solution of 1.145 g (16.6
mmol) of sodium nitrite in 40 ml of water are added simultaneously to 18 ml
of concentrated hydrochloric acid at -5°C with stirring. The resulting solution
(about 25°C) is red colored and on cooling to 0°C a crystalline solid begins to
separate. After 10 minutes at 0°C, an ice-cold solution of 1.057 g (16.25
mmol) of sodium azide in 40 ml of water is added in portions. A cream
colored precipitate forms immediately, accompanied by copious evolution of
nitrogen. The reaction is completed when no more red color is visible. If
necessary, a further little excess of NaN3 solution may be added. Stirring is
continued for 10 minutes at 0°C and then the mixture is extracted with
ethylacetate. The organic phase is separated, washed with water, dried over
Na 2 SO 4 and concentrated in vacuum to give 5.67 g (100%) of 1-(p-
azidobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid. Thin layer
chromatography on silica gel gives one spot in the system chloroform-ethanol 95:5. An analytical sample is obtained by crystallization from methanol-water:
MP: 170-172°C (with gas evolution). The structure of zidometacin is
confirmed by IR spectrum.
The starting compounds were synthesized next way:
Preparation of methyl 1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3- indolylacetate:
To a solution of 23.3 g (0.1 mole) of methyl-5-methoxy-2-methyl-3- indolylacetate in 50 ml of dry toluene are added 3 g of 80% sodium hydride. The mixture is stirred at room temperature for 4 hours and then a solution of 18.56 g (0.1 mole) of p-nitrobenzoylchloride in 80 ml of dry toluene is added slowly thereto over a 30-minute period. The reaction mixture is boiled for 30 hours. After cooling it is poured into 400 ml of ice-water and 15 ml of acetic acid. The separated toluene solution is washed with a large quantity of water, dried over sodium sulfate and evaporated to a syrup which is dissolved in ether. Slow evaporation of this solution in an open beaker gives 10 g of methyl-1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-indolylacetate as yellow prisms. Another quantity may be recovered from the oily residue after chromatography on a silica gel column (elution with benzene). MP: 134-135°C (crystallization from MeOH).
Preparation of 1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid:
A solution of 4.9 g (12.8 mmol) of methyl-1-(p-nitrobenzoyl)-5-methoxy-2- methyl-3-indolylacetate in 40 ml of acetic acid containing 400 mg of p- toluene-sulfonic acid is refluxed for 20 hours and then concentrated in vacuum. The gummy residue is extracted with ethyl acetate. The extract is filtered from insoluble material, washed with water and dried over sodium sulfate. Removal of the solvent under reduced pressure affords the desired product as yellow crystals; MP: 185-186°C.
Preparation of 1-(p-aminobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid:
20 g (54.3 mmol) of 1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid is dissolved in 1200 ml of hot methanol and hydrogenated in the presence of 2.64 g of 10% palladium on charcoal as catalyst. After 164 mmol of hydrogen have been consumed, the hydrogenation is stopped, and the solution filtered to remove the catalyst. The filtrate is concentrated in vacuum to give, in nearly theoretical yield, the title p-amino derivative. A crystallization from methanol-water gave an analytical sample: MP 198-200°C (dec.) crystals from MeOH-H 2 O.
The starting compounds were synthesized next way:
Preparation of methyl 1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3- indolylacetate:
To a solution of 23.3 g (0.1 mole) of methyl-5-methoxy-2-methyl-3- indolylacetate in 50 ml of dry toluene are added 3 g of 80% sodium hydride. The mixture is stirred at room temperature for 4 hours and then a solution of 18.56 g (0.1 mole) of p-nitrobenzoylchloride in 80 ml of dry toluene is added slowly thereto over a 30-minute period. The reaction mixture is boiled for 30 hours. After cooling it is poured into 400 ml of ice-water and 15 ml of acetic acid. The separated toluene solution is washed with a large quantity of water, dried over sodium sulfate and evaporated to a syrup which is dissolved in ether. Slow evaporation of this solution in an open beaker gives 10 g of methyl-1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-indolylacetate as yellow prisms. Another quantity may be recovered from the oily residue after chromatography on a silica gel column (elution with benzene). MP: 134-135°C (crystallization from MeOH).
Preparation of 1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid:
A solution of 4.9 g (12.8 mmol) of methyl-1-(p-nitrobenzoyl)-5-methoxy-2- methyl-3-indolylacetate in 40 ml of acetic acid containing 400 mg of p- toluene-sulfonic acid is refluxed for 20 hours and then concentrated in vacuum. The gummy residue is extracted with ethyl acetate. The extract is filtered from insoluble material, washed with water and dried over sodium sulfate. Removal of the solvent under reduced pressure affords the desired product as yellow crystals; MP: 185-186°C.
Preparation of 1-(p-aminobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid:
20 g (54.3 mmol) of 1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid is dissolved in 1200 ml of hot methanol and hydrogenated in the presence of 2.64 g of 10% palladium on charcoal as catalyst. After 164 mmol of hydrogen have been consumed, the hydrogenation is stopped, and the solution filtered to remove the catalyst. The filtrate is concentrated in vacuum to give, in nearly theoretical yield, the title p-amino derivative. A crystallization from methanol-water gave an analytical sample: MP 198-200°C (dec.) crystals from MeOH-H 2 O.
Preparation Products And Raw materials
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine