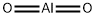Aluminum oxide is an electrical insulator but has a relatively high thermal conductivity for a ceramic material. It is thus used as insulating material in power electronics. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Since metallic aluminum is very reactive with atmospheric oxygen, a thin passivation layer of alumina (4 nm thickness) forms on any exposed aluminum surface, protecting the metal from further oxidation.
Corundum is the most common naturally occurring crystalline form of aluminium oxide.Rubies and sapphires are gem-quality forms of corundum, which owe their characteristic colours to trace impurities. Rubies are given their characteristic deep red colour and their laser qualities by traces of chromium.Sapphires come in different colours given by various other impurities, such as iron and titanium. An extremely rare δ form occurs as the mineral deltalumite.
Aluminium Oxide is used for the separation of both inorganic anions and acidic organic molecules such as acidic amino acids, aromatic acids and carboxylic acids. It is essential in protein extraction as a component in the preparation of tissues. It is also used as a grinding or blending agent. Aluminum oxide is a source of aluminum in reactions, an abrasive agent, and as a refractory material.
Chronic inhalation of Al2O3 dusts may cause lung damage.
Structure and conformation
The most common form of crystalline aluminium oxide is known as corundum, which is the thermodynamically stable form.The oxygen ions form a nearly hexagonal close-packed structure with the aluminium ions filling two-thirds of the octahedral interstices. Each Al3+ center is octahedral. In terms of its crystallography, corundum adopts a trigonal Bravais lattice with a space group of R3c.