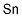Tin Metal is a ductile, malleable, lustrous solid with a gray to almost silver-white color. Tin forms covalent bonds with carbon forming a variety of organometallic or organotin compounds, and the physical and chemical properties differ for various compounds such as Tin II (SnO) and Tin IV (SnO2), organotins, and inorganic tin compounds. Tin Metal is not easily oxidized and resists corrosion; however, powdered tin has a tendency to oxidize, especially at high humidity.

Tin Metal is a silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort.Tin Metal is generally insoluble in water and have vapor pressures of approximately 0mmHg.
Although tin metal is located in group 14 as a metalloid, it retains one of the main characteristicsof metals: in reacting with other elements, it gives up electrons, forming positive ions just asdo all metals.
Tin Metal has a relatively low melting point (about 231°C or 4,715°F), and it reacts with someacids and strong alkalis, but not with hot water. Its resistance to corrosion is the main characteristicthat makes it a useful metal.
Tin metal-plating of iron protects the latter from corrosion; tin piping and valves maintain purity in water and beverages; molten tin is the base for (float) plate-glass production. Because pure tin metal is relatively weak, it is not put to structural uses unless alloyed with other metals in such materials as bronzes, pewter, bearing metals, type metals, lead-based solders, bell metal, babbitt metal, and low-temperature casting alloys. Tin oxide, in which tin is in the +4 oxidation state, is useful in making ceramic bodies opaque, as a mild abrasive, and as a weighting agent for fabrics. Tin fluoride and tin pyrophosphate, in which tin is in the +2 oxidation state, are used in dentifrices. Organic tin compounds act as stabilizers in certain plastics and as wood preservatives.
At ordinary temperatures tin is stable in air. It actually forms a very thin protective oxide film. In powder form, and especially in the presence of moisture, it oxidizes. When heated with oxygen it forms tin(IV) oxide, SnO2.Tin reacts with all halogens forming their halides. Reaction with fluorine is slow at ordinary temperatures; however, chlorine, bromine and iodine readily react with the metal.
Tin is attacked by concentrated acids. With dilute acids the reaction may be slow or very slow. The metal readily reacts with hot concentrated hydrochloric acid and aqua regia but slowly with cold dilute hydrochloric acid. The reaction also is slow with hot dilute sulfuric acid, which dissolves the metal, particularly in the presence of an oxidizing agent. The reaction with nitric acid is generally slow. Hot concentrated acid converts the metal to an insoluble hydrated tin(IV) oxide. The reaction is rapid with moist sulfur dioxide or sulfurous acid, chlorosulfonic, and pyrosulfuric acids.
Tin Metal is apparently nontoxic, and quantities of tin up to 300 parts per million, as dissolved by foods packaged in tin-plated containers and cooking utensils, are not harmful. Organic tin compounds commonly used as biocides and fungicides are, however, toxic to human beings.