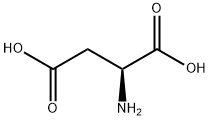
Aspartate is a non-essential amino acid, meaning that it is readily and naturally synthesized by mammals. It is one of the 20 building-block amino acids of proteins, 3-letter code is ASP, one letter code is D. The DNA codons encoding aspartic acid are GAC and GAU.
In proteins aspartate sidechains are often hydrogen bonded to form asx turns or asx motifs, which frequently occur at the N-termini of alpha helices.Aspartate, like glutamic acid, is classified as an acidic amino acid, with a pKa of 3.9; however, in a peptide this is highly dependent on the local environment, and could be as high as 14.
Aspartate is used as an electrolyte supplement for aminophenol transfusion, inorganic ion supplement (K+, Ca+, etc.) and fatigue restorer. Potassium magnesium aspartate injection or oral solution can be used for arrhythmia, premature beat, tachycardia, hypokalemia, hypomagnesemia, heart failure, myocardial infarction, angina pectoris, hepatitis, cirrhosis and other diseases caused by cardiac glycoside poisoning.
Industrially, aspartate is produced by amination of fumarate catalyzed by L-aspartate ammonia-lyase.Racemic aspartic acid can be synthesized from diethyl sodium phthalimidomalonate, (C6H4(CO)2NC(CO2Et)2).
There are two forms or enantiomers of aspartic acid. Of these two forms, only one, "L-aspartic acid", is directly incorporated into proteins. The biological roles of its counterpart, "D-aspartic acid" are more limited. Where enzymatic synthesis will produce one or the other, most chemical syntheses will produce both forms, "DL-aspartic acid", known as a racemic mixture.