Bicine is an amino acid that results from amine degradation due to the presence of oxygen and/or sulfur dioxide. The amine solvents that undergo this degradation are MDEA, DEA, TEA, and mixed amine solvents containing any of these amines. As a zwitterionic amino acid buffer agent, bicine is active in the pH 7.6-9.0 range (pKa of 8.26 at 25°C). Bicine is a recommended buffer for low-temperature biochemical work. It is used for the preparation of stable substrate solution for serum guanase determination. It can be used as a nitrogenous strength enhancer to regulate cement hydration and improve cement properties. Studies have found that bicine significantly changes the morphology of portlandite crystals from a parallel-stacked lamellar shape to a distorted one and also decreases the pore size of the hardened cement paste at an early age[1]. In particular, bicine could promote the rapid formation of β-sheet-rich amyloid-β fibrils[2].
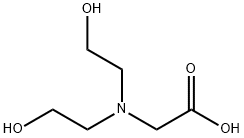
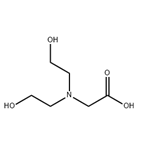
Bicine is a white/clear crystalline powder that is somewhat soluble in water. It contains an amino group, carboxyl group, and two hydroxyl units in its structure, making it highly polar. It is often used as a biological buffer and chelating agent.
To prepare stable substrate solution for the determination of guanine deaminase. The buffer is widely used with the working concentration of 3-100mM.
A zwitterionic amino acid buffer agent used for low temperature work. The use of bicine in a thin layer ion exchange chromatography method for protein resolution has been published. Bicine has been utilized in peptide and protein crystallization. A kinetic study of a quaternary transition-state analogue complex of creatine kinase used bicine in the reaction buffer. A multiphasic buffer system for SDS-PAGE of proteins and peptides that incorporates bicine has been described.
ChEBI: N,N-bis(2-hydroxyethyl)glycine is a bicine that is a Good's buffer substance, pKa = 8.35 at 20 ℃. It is a conjugate acid of a [bis(2-hydroxyethyl)amino]acetate. It is a tautomer of a [bis(2-hydroxyethyl)ammonio]acetate.
Dissolve bicine in a small volume of hot water and precipitate it with EtOH, twice. Repeat once more but treat the aqueous solution with charcoal Purification of Biochemicals — Amino Acids and Peptides and filter before adding EtOH. Also crystallise it from concentrated aqueous solutions. [Torn & Kolthoff J Am Chem Soc 77 2061 1955, Chaberek et al. J Am Chem Soc 75 2185 1953, Beilstein 4 IV 2390.]
[1] Lu X, et al. The influence of bicine on the hydration and properties of Portland cement. Journal of Thermal Analysis and Calorimetry, 2022; 147: 13125–13134.
[2] Kim H, et al. Bicine promotes rapid formation of β-sheet-rich amyloid-β fibrils. PLOS ONE, 2020.