Liquid Chromatography
Liquid chromatography is currently one of the important methods for analysis of trace amount of substance component. The two-phase in the chromatographic analysis refers to immobile phase having a large specific surface area and mobile phase carrying mixture to be separated to flow through the immobile phase. Chromatography using liquid as mobile phase is called as liquid chromatography.
Liquid chromatography is one kind of chromatography method with the adopted mobile phase being liquid. Owing to the liquid contained in the column gets higher resistance than gas in the column, in order to increase the speed of analysis, people usually adopt high-pressure fluid way, so called "high-pressure liquid chromatography." According to the difference of the mobile phase, it can be divided into liquid - solid chromatography and liquid - liquid chromatography; according to the difference type of equilibrium of the immobile phase and the mobile phase, it can be divided into adsorption chromatography, partition chromatography, ion exchange chromatography and gel chromatography. It can be used for the separation and analysis of organic, inorganic and polymer liquid.
Brief History
In 1906, the Russian botanist Tswett has for the first time applied column chromatography for the separation plant pigments, and is one of oldest liquid chromatography. In the recent 30 years, people have developed various kinds of chromatography and become an important part of the chemical analysis.
① classical liquid chromatography: mobile phase flow downward from the upper end of the column tube through its own gravity; collect the effluent one tube by tube from the lower portion of the column at the outlet and then apply other methods of measurement with slow analysis rate and low efficiency;
② Thin-layer chromatography : it appeared in around 1940s, easy to operate and have obtained improvement on the speed of analysis, but has poor reproducibility and difficult for quantitative analysis;
③ High performance liquid chromatography: after the early 1960s, J. C. Gidelings had amended the gas chromatography theory to liquid chromatography and had laid the foundation for its modernization. Thereafter, technically people had used high-pressure pump, high efficiency immobile phase and a high sensitivity detector and had developed the high performance liquid chromatography with high speed analysis, high separation efficiency and automatic operation. In 1971, R. A. Henry has first applied it for pesticide analysis.
High performance liquid chromatography
High performance liquid chromatography is one of the most important separation method of modern analytical chemistry. It is originated in the classical liquid chromatography with the basic approach as below: use single solvent of certain polarity or mixed solution of different proportions as mobile phase; pump the mobile phase into the column equipped with a filler; after the injected tested sample has been brought into the column by the mobile phase, each components then entered the detector one after another; use recorder or data processing device to record the chromatogram or perform data processing to obtain the measurement results. Owing to the application of the filler particles of various characteristics and pressurized liquid mobile phase, the separation method has high performance and rapid analysis features.
High performance liquid chromatography analysis is suitable for the determination and measurement of the drugs which can be separated on column equipped with certain filler, in particular for the determination of multi-component drugs, impurities inspection and the determination of macromolecules. Some drugs need to be subject to derivatization reaction prior to or after the chromatographic separation in order to be isolated or detected. The commonly used filler of the column include: silica gel used for normal phase chromatography; chemically bonded immobile phase, depending on the bonding groups, can be used in either reverse phase or normal phase chromatography; ion exchange filler is used for ion exchange chromatography; macroporous filler with certain aperture is used for exclusion chromatography.
High performance liquid chromatography consists of the basic pump, injector, column, detector and the processing system of the chromatographic data. The most commonly used detector is for detecting UV and visible light; other detectors also include refractive index detector and evaporative light scattering detector and so on. The collection and processing of the chromatography information often proceed in information workstation and integrators. Gradient elution can be obtained through program control with two pumps or single pump plus proportional valve.
- Structure:
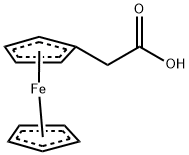
- Chemical Name:FERROCENEACETIC ACID
- CAS:1287-16-7
- MF:C12H12FeO2
- Structure:
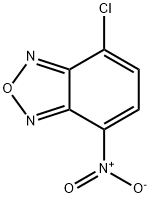
- Chemical Name:4-Chloro-7-nitrobenzofurazan
- CAS:10199-89-0
- MF:C6H2ClN3O3
- Structure:
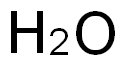
- Chemical Name:Water
- CAS:7732-18-5
- MF:H2O
- Structure:
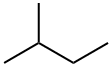
- Chemical Name:2-Methylbutane
- CAS:78-78-4
- MF:C5H12
- Structure:
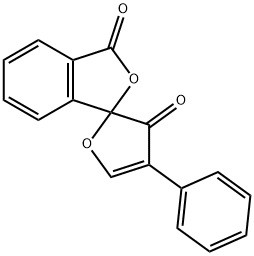
- Chemical Name:Fluorescamine
- CAS:38183-12-9
- MF:C17H10O4
- Structure:
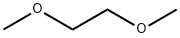
- Chemical Name:1,2-Dimethoxyethane
- CAS:110-71-4
- MF:C4H10O2
- Chemical Name:PETROLEUM ETHER
- CAS:8032-32-4
- MF:unspecified
- Structure:
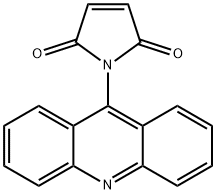
- Chemical Name:NAM
- CAS:49759-20-8
- MF:C17H10N2O2
- Structure:
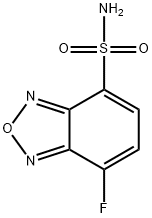
- Chemical Name:ABD-F
- CAS:91366-65-3
- MF:C6H4FN3O3S
- Structure:
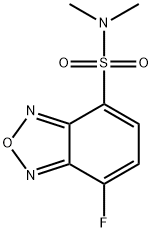
- Chemical Name:4-(N,N-DIMETHYLAMINOSULFONYL)-7-FLUORO-2,1,3-BENZOXADIAZOLE
- CAS:98358-90-8
- MF:C8H8FN3O3S
- Structure:
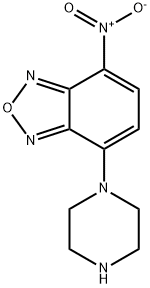
- Chemical Name:4-Nitro-7-(1-piperazinyl)-2,1,3-benzoxadiazole
- CAS:139332-66-4
- MF:C10H11N5O3
- Structure:
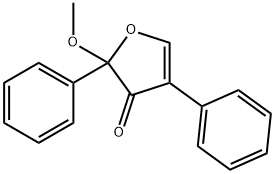
- Chemical Name:2-METHOXY-2,4-DIPHENYL-3(2H)-FURANONE
- CAS:50632-57-0
- MF:C17H14O3
- Structure:
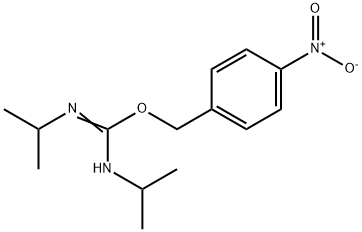
- Chemical Name:O-(4-NITROBENZYL)-N,N'-DIISOPROPYLISOUREA
- CAS:2978-11-2
- MF:C14H21N3O3
- Structure:
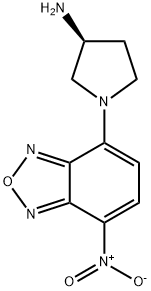
- Chemical Name:(S)-(+)-4-NITRO-7-(3-AMINOPYRROLIDIN-1-YL)-2,1,3-BENZOXADIAZOLE
- CAS:143112-52-1
- MF:C10H11N5O3
- Structure:
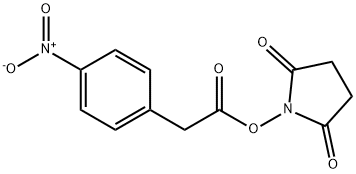
- Chemical Name:N-SUCCINIMIDYL 4-NITROPHENYLACETATE
- CAS:68123-33-1
- MF:C12H10N2O6
- Structure:
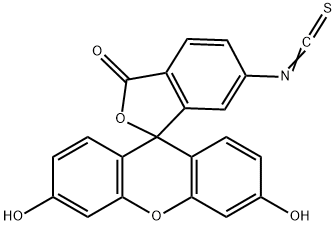
- Chemical Name:Fluorescein 6-isothiocyanate
- CAS:18861-78-4
- MF:C21H11NO5S
- Structure:
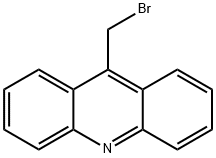
- Chemical Name:9-(BROMOMETHYL)ACRIDINE
- CAS:1556-34-9
- MF:C14H10BrN
- Structure:
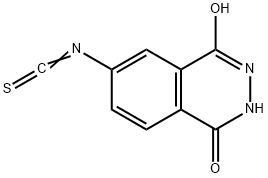
- Chemical Name:2,3-DIHYDRO-6-ISOTHIOCYANATO-1,4-PHTHALAZINEDIONE
- CAS:107807-39-6
- MF:C9H5N3O2S
- Structure:
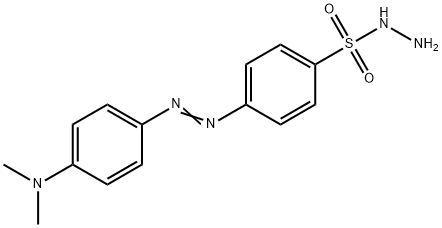
- Chemical Name:DABSYL HYDRAZINE
- CAS:72565-41-4
- MF:C14H17N5O2S
- Structure:
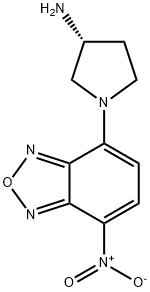
- Chemical Name:(R)-(-)-NBD-APY
- CAS:143112-51-0
- MF:C10H11N5O3
- Structure:
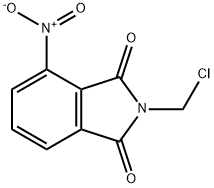
- Chemical Name:N-CHLOROMETHYL-4-NITROPHTHALIMIDE
- CAS:54455-34-4
- MF:C9H5ClN2O4
- Structure:
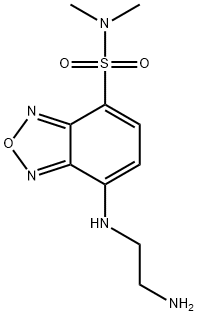
- Chemical Name:DBD-ED
- CAS:189373-41-9
- MF:C10H15N5O3S
- Structure:
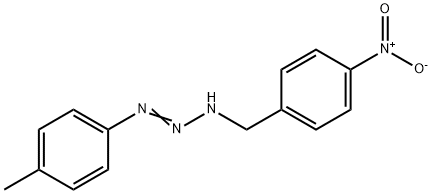
- Chemical Name:1-(4-NITROBENZYL)-3-P-TOLYLTRIAZENE
- CAS:60259-80-5
- MF:C14H14N4O2
- Structure:
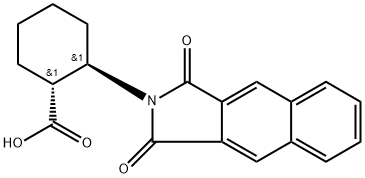
- Chemical Name:(1R,2R)-2-(NAPHTHALENE-2,3-DICARBOXIMIDO)CYCLOHEXANECARBOXYLIC ACID
- CAS:642995-15-1
- MF:C19H17NO4
- Structure:
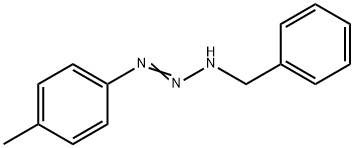
- Chemical Name:1-BENZYL-3-P-TOLYLTRIAZENE
- CAS:17683-09-9
- MF:C14H15N3
- Structure:
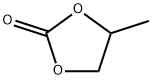
- Chemical Name:108-32-7
- CAS:108-32-7
- MF:
- Structure:
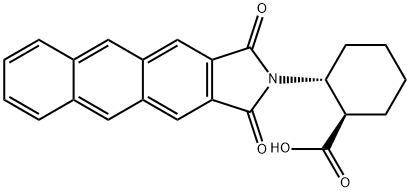
- Chemical Name:(1R,2R)-2-(ANTHRACENE-2,3-DICARBOXIMIDO)CYCLOHEXANECARBOXYLIC ACID
- CAS:446044-44-6
- MF:C23H19NO4
- Structure:
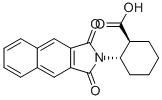
- Chemical Name:(1S,2S)-2-(NAPHTHALENE-2,3-DICARBOXIMIDO)CYCLOHEXANECARBOXYLIC ACID
- CAS:642995-16-2
- MF:C19H17NO4
- Structure:

- Chemical Name:4-(N,N-DIMETHYLAMINOSULFONYL)-7-[5-(SUCCINIMIDYLOXYCARBONYL)PENTYLAMINO]-2,1,3-BENZOXADIAZOLE
- CAS:1858255-08-9
- MF:C18H23N5O7S
- Structure:
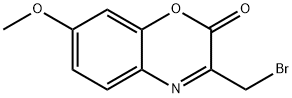
- Chemical Name:3-BROMOMETHYL-7-METHOXY-1,4-BENZOXAZIN-2-ONE
- CAS:124522-09-4
- MF:C10H8BrNO3
- Structure:

- Chemical Name:(S)-(-)-NBD-PRO-COCL
- CAS:
- MF:C11H9ClN4O4
- Structure:
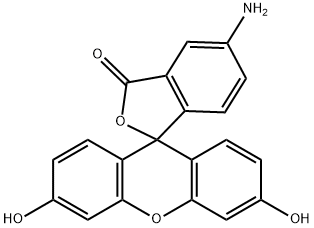
- Chemical Name:5-Aminofluorescein
- CAS:3326-34-9
- MF:C20H13NO5
- Structure:
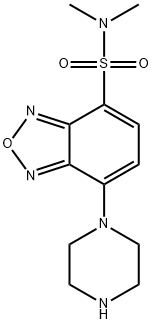
- Chemical Name:DBD-PZ
- CAS:139332-64-2
- MF:C12H17N5O3S
- Structure:
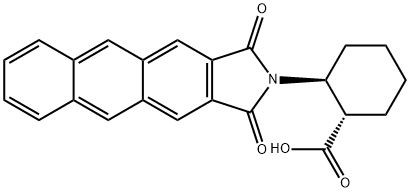
- Chemical Name:(1S,2S)-2-(ANTHRACENE-2,3-DICARBOXIMIDO)CYCLOHEXANECARBOXYLIC ACID
- CAS:446044-45-7
- MF:C23H19NO4
- Structure:
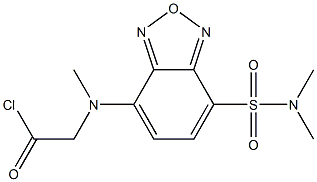
- Chemical Name:DBD-COCL
- CAS:156153-43-4
- MF:C11H13ClN4O4S
- Structure:

- Chemical Name:4-(5-CARBOXYPENTYLAMINO)-7-(N,N-DIMETHYLAMINOSULFONYL)-2,1,3-BENZOXADIAZOLE
- CAS:1820741-40-9
- MF:C14H20N4O5S
- Structure:
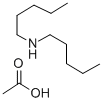
- Chemical Name:DIPENTYLAMINE ACETATE SOLUTION
- CAS:211676-91-4
- MF:C12H27NO2
- Structure:
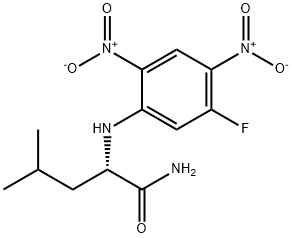
- Chemical Name:NALPHA-(5-FLUORO-2,4-DINITROPHENYL)-L-LEUCINAMIDE
- CAS:178065-29-7
- MF:C12H15FN4O5
- Chemical Name:DMEM
- CAS:
- MF:
- Structure:
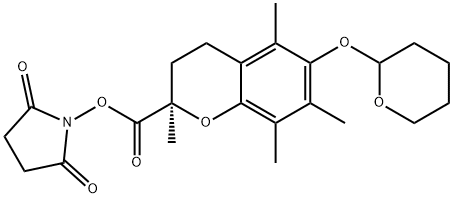
- Chemical Name:Succinimidyl (2R)-6-(Tetrahydro-2H-pyran-2-yloxy)-2,5,7,8-tetramethylchroman-2-carboxylate
- CAS:1069137-73-0
- MF:C23H29NO7
- Structure:
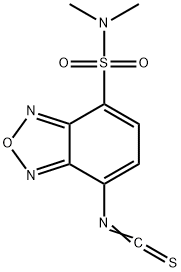
- Chemical Name:DBD-NCS
- CAS:147611-81-2
- MF:C9H8N4O3S2
- Structure:
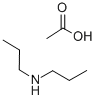
- Chemical Name:DIPROPYLAMINE ACETATE SOLUTION
- CAS:114389-69-4
- MF:C8H19NO2
- Structure:
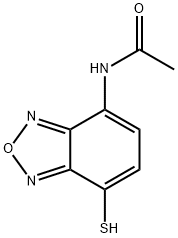
- Chemical Name:AABD-SH
- CAS:254973-02-9
- MF:C8H7N3O2S
- Structure:
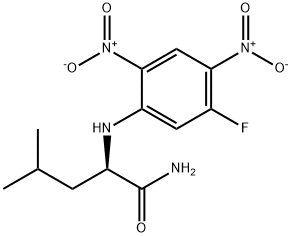
- Chemical Name:NALPHA-(5-FLUORO-2,4-DINITROPHENYL)-D-LEUCINAMIDE
- CAS:178065-30-0
- MF:C12H15FN4O5
- Structure:
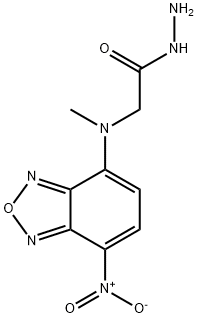
- Chemical Name:NBD-CO-HZ
- CAS:221263-97-4
- MF:C9H10N6O4
- Structure:
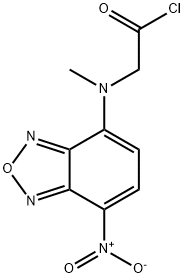
- Chemical Name:NBD-COCL
- CAS:140164-85-8
- MF:C9H7ClN4O4
- Structure:
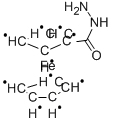
- Chemical Name:(HYDRAZINOCARBONYL)FERROCENE
- CAS:12153-28-5
- MF:C11H12FeN2O10*
- Structure:
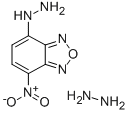
- Chemical Name:NBD-H
- CAS:131467-87-3
- MF:C6H9N7O3
- Structure:
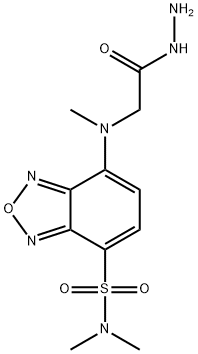
- Chemical Name:DBD-CO-HZ
- CAS:179951-63-4
- MF:C11H16N6O4S
- Structure:
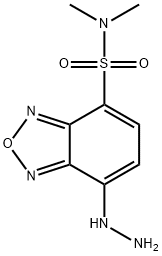
- Chemical Name:DBD-H
- CAS:131467-86-2
- MF:C8H11N5O3S
- Structure:

- Chemical Name:(R)-(+)-NBD-PRO-COCL
- CAS:
- MF:C11H9ClN4O4