Organosilicon compounds
In 1863, two scientists C. Friedel and J.M. Crafts had synthesized organic silicon compound tetraethyl silane (C2H5)4Si for the earliest time. Later, in 1945, there had been direct synthesis of chlorosilanes in the presence of a catalyst, establishing the foundation of modern organic silicon industry. Latter it had appeared of applying hydrosilation reaction for generation of organic silicone in 1947 and thermal condensation production of organic silicon monomer in 1950. All of this had created the conditions for the production of organic silicon with a functional group.
Organosilicon compound referred to as "organic silicon", and is one important type in the organic compound of elements, refers to the organic compound containing carbon - silicon bond inside the molecule structure. It has similarity in its structure with the organic carbon compounds but also has difference with organic carbon compounds. For example, the silicone molecule silicon - only contains single bond between silicon atoms without containing double and triple bonds. Simple types contain halosilanes (e.g. CH3SiCl3), silanol [such as (CH3) 3SiOH] and silyl ethers [e.g., (CH3) 3Si- O-Si (CH3) 3] and so on. It is customarily also classify certain compounds containing silicon element such as TEOS [(C2H5O) 4Si], monosilane (SiH4) as organic silicon compounds. Such compounds have special properties, such as being able to be polymerized into polysiloxane (the formula of the simplest polymer is R3Si (OSiR2) n OSiR3), having heat resistance, water resistance and excellent electrical insulation properties and being able to used for manufacturing of silicone oil, silicone rubber and silicone resin.
At room temperature, most of the organic silicon compound appears as colorless transparent liquids with a certain toxicity and corrosiveness. It is soluble in many organic solvents and prone to burn and produce toxic fumes in case of fire, heat and oxidants. Phenylsilane, upon contact with water and steam, can produce toxic and corrosive gases. In industry, it is mainly used as reagents, water repellent, silicide intermediates and synthesis of polymer organic silicide. In the management of dangerous goods, alkyl silicide is typically used as flammable liquids; phenyl silicide is classified into corrosive substances. In case of fire of phenyl silicide, we should not use water or foam to extinguish; instead we can use foam, dry powder, 1211, and carbon dioxide as well as water spray for extinguishing.
Introduction of the major applications of the organic silicon compound:
Organosilicon compounds can be applied directly in the form of monomeric compounds; it can also be made into silicone polymer through hydrolysis and polymerization. As a kind of monomer, it can be used as the silylating agent as well as being used for covering the surface of the material, moisture protection of the electrically insulating material, anti-adhesive of particulate material, organic synthesis and materials’ separation; as a coupling agent, it can be used for improving the adhesion between resin or rubber and other materials, improving the mechanical properties of plastics and rubber and waterproof performance; it can be used as a paint additive; it can also be applied to drugs of boosting hair growth and anti-cancer drugs. When being applied in the form of polymer, it can be used for preparation of silicone oil (polydimethylsiloxane), used in cosmetics, lubricants, defoamers, water repellent, leather softener, release agents, heat transfer media, and hydraulic oil; it can also be used for insulating paint, filler, inorganic fiber binders, waterproofing agents, anti-corrosion coatings in the form of silicon resin; when being in the form of silicon rubber, it can be used for the anti-aging elastic material; at room temperature, the vulcanizing silicon rubber can be used for potting materials and pressure molding accessory; it can be applied to gas chromatography and mass spectrometry; the most important category in industry is methyl and phenyl chlorosilane, such as a chlorosilane; in particular, trimethylchlorosilane plays an important role in organic synthesis and biochemistry; the methyl polysiloxane is an important component of cosmetics and hair care agents as well as anti-foaming agent. Apart from the above applications, organic silicon compounds, in the field of organic synthesis, can also be used as a reducing agent, protection agent of the active group and the substrate of selective electrophilic substitution reaction as well as Peterson reagents.
- Structure:
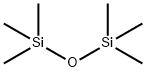
- Chemical Name:Hexamethyldisiloxane
- CAS:107-46-0
- MF:C6H18OSi2
- Structure:
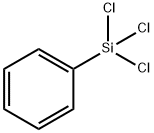
- Chemical Name:Phenyltrichlorosilane
- CAS:98-13-5
- MF:C6H5Cl3Si
- Structure:
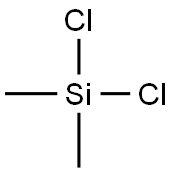
- Chemical Name:Dichlorodimethylsilane
- CAS:75-78-5
- MF:C2H6Cl2Si
- Structure:
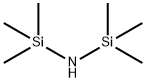
- Chemical Name:Hexamethyldisilazane
- CAS:999-97-3
- MF:C6H19NSi2
- Structure:
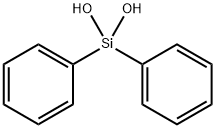
- Chemical Name:Diphenylsilanediol
- CAS:947-42-2
- MF:C12H12O2Si
- Structure:
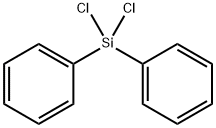
- Chemical Name:Dichlorodiphenylsilane
- CAS:80-10-4
- MF:C12H10Cl2Si
- Structure:
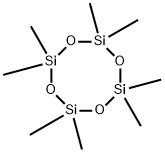
- Chemical Name:Octamethylcyclotetrasiloxane
- CAS:556-67-2
- MF:C8H24O4Si4
- Structure:
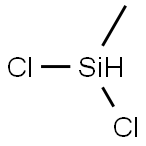
- Chemical Name:Dichloromethylsilane
- CAS:75-54-7
- MF:CH4Cl2Si
- Structure:
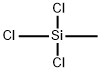
- Chemical Name:Methyltrichlorosilane
- CAS:75-79-6
- MF:CH3Cl3Si
- Structure:
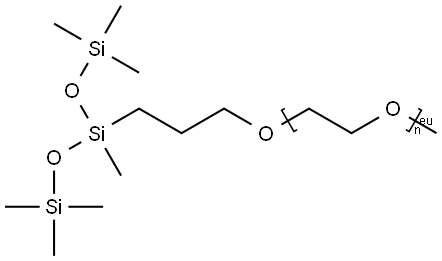
- Chemical Name:Glycols, polyethylene, methyl 3-[1,3,3,3-tetramethyl-1-(trimethylsiloxy)disiloxanyl]propyl ether
- CAS:27306-78-1
- MF:(C2H4O)nC11H30O3Si3
- Structure:
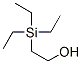
- Chemical Name:2-(Triethylsilyl)ethanol
- CAS:2916-67-8
- MF:
- Structure:
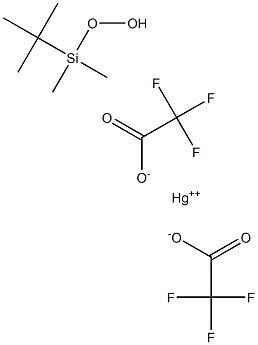
- Chemical Name:t-Butyldimethylsilyl Hydroperoxide-Mercury(II) Trifluoroacetate
- CAS:78957-19-4
- MF:C10H16F6HgO6Si
- Structure:

- Chemical Name:3-(Methoxymethylene)-2,4-bis(trimethylsilyloxy)-1,4-pentadiene
- CAS:90330-49-7
- MF:C13H26O3Si2
- Structure:
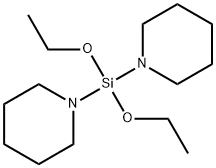
- Chemical Name:Diethoxy-Di(Piperidin-1-Yl)Silane
- CAS:1353744-75-8
- MF:C14H30N2O2Si
- Structure:
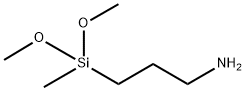
- Chemical Name:3-(Dimethoxymethylsilyl)propylamine
- CAS:3663-44-3
- MF:C6H17NO2Si
- Chemical Name:Bind-Silane
- CAS:
- MF:
- Structure:
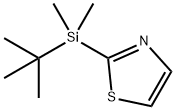
- Chemical Name:2-(tert-Butyldimethylsilyl)thiazole
- CAS:137382-38-8
- MF:C9H17NSSi
- Chemical Name:Vinyl terminated polydimethylsiloxane
- CAS:68083-19-2
- MF:N/A
- Structure:
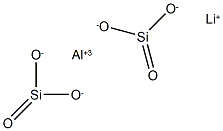
- Chemical Name:spodumene
- CAS:1302-37-0
- MF:AlLiO6Si2
- Structure:
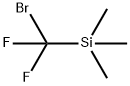
- Chemical Name:TriMethyl(broModifluoroMethyl)silane
- CAS:115262-01-6
- MF:C4H9BrF2Si
- Structure:

- Chemical Name:Bromobis(isopropylthio)borane
- CAS:89449-88-7
- MF:C6H14BBrS2
- Structure:
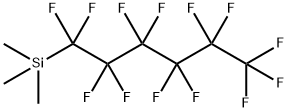
- Chemical Name:Silane, trimethyl(tridecafluorohexyl)-
- CAS:135841-49-5
- MF:C9H9F13Si
- Structure:
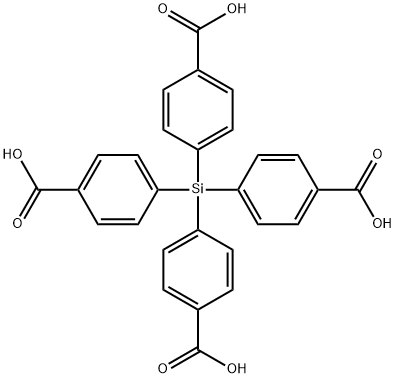
- Chemical Name:tetrakis(4-carboxyphenyl)silane
- CAS:10256-84-5
- MF:C28H20O8Si
- Structure:

- Chemical Name:Sodium 5-[(4-Amino-1-naphthyl)azo]-2-[4-[(2-hydroxy-1-naphthyl)azo]-2-sulfonatostyryl]benzenesulfonate
- CAS:6459-79-6
- MF:C34H26N5NaO7S2
- Structure:
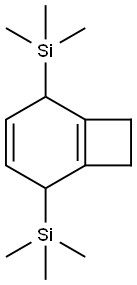
- Chemical Name:Silane, bicyclo[4.2.0]octa-1(6),3-diene-2,5-diylbis[trimethyl-
- CAS:145708-70-9
- MF:C14H26Si2
- Structure:
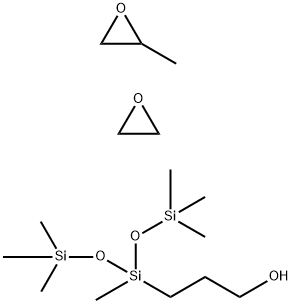
- Chemical Name:Oxirane, methyl-, polymer with oxirane, mono3-1,3,3,3-tetramethyl-1-(trimethylsilyl)oxydisiloxanylpropyl ether
- CAS:134180-76-0
- MF:C15H38O5Si3
- Structure:
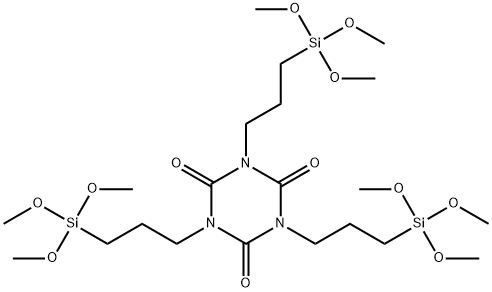
- Chemical Name:Tris[3-(trimethoxysilyl)propyl] Isocyanurate
- CAS:26115-70-8
- MF:C21H45N3O12Si3
- Structure:
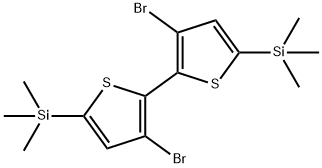
- Chemical Name:3,3'-Dibromo-5,5'-bis(trimethylsilyl)-2,2'-bithiophene
- CAS:207742-50-5
- MF:C14H20Br2S2Si2
- Structure:
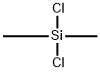
- Chemical Name:POLY(DIMETHYLSILANE)
- CAS:30107-43-8
- MF:C2H6Cl2Si
- Structure:
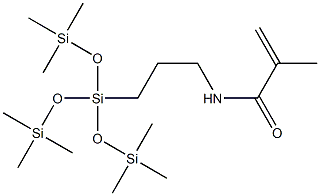
- Chemical Name:3-Methacrylamidopropyl Tris(Trimethylsiloxy)Silane
- CAS:115257-95-9
- MF:C16H39NO4Si4
- Structure:
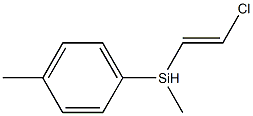
- Chemical Name:Silane, chloroethenylmethyl(4-methylphenyl)-
- CAS:41924-54-3
- MF:C10H13ClSi
- Chemical Name:TRIS BUFFERED SALINE
- CAS:
- MF:
- Structure:
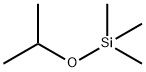
- Chemical Name:ISOPROPOXYTRIMETHYLSILANE
- CAS:1825-64-5
- MF:C6H16OSi
- Chemical Name:Siloxanes and Silicones, 3-(2-aminoethyl)aminopropyl Me, di-Me, methoxy-terminated
- CAS:102782-92-3
- MF:
- Structure:
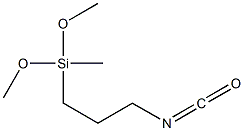
- Chemical Name:Silane,(3-isocyanatopropyl)dimethoxymethyl
- CAS:26115-72-0
- MF:C7H15NO3Si
- Structure:

- Chemical Name:DIMETHYLSILOXANE, PROPYLENE OXIDE/ETHYLENE OXIDE BLOCK COPOLYMER
- CAS:68937-55-3
- MF:C2H10OSi2
- Structure:
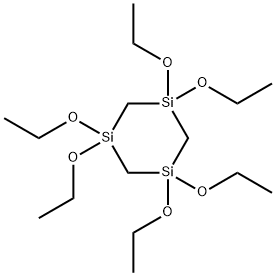
- Chemical Name:1,1,3,3,5,5-hexaethoxy-1,3,5-trisilacyclohexane
- CAS:17955-67-8
- MF:C15H36O6Si3
- Chemical Name:SILICONE OV-101
- CAS:
- MF:
- Structure:
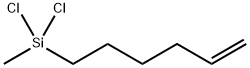
- Chemical Name:5-Hexenylmethyl dichlorosilane
- CAS:90054-19-6
- MF:C7H14Cl2Si
- Structure:
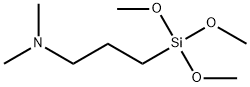
- Chemical Name:(N,N-Dimethylaminopropyl)trimethoxysilane
- CAS:2530-86-1
- MF:C8H21NO3Si
- Structure:
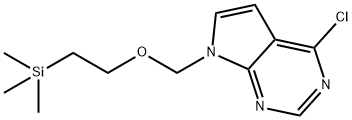
- Chemical Name:4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE
- CAS:941685-26-3
- MF:C12H18ClN3OSi
- Structure:
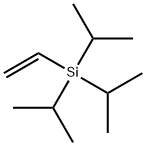
- Chemical Name:ethenyl-tri(propan-2-yl)silane
- CAS:121675-48-7
- MF:C11H24Si
- Structure:
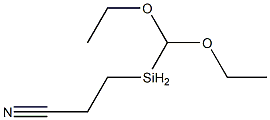
- Chemical Name:2-CYANOETHYLMETHYLDIETHOXYSILANE
- CAS:1186-11-4
- MF:C8H17NO2Si
- Structure:

- Chemical Name:Dimethylpolysilane
- CAS:28883-63-8
- MF:(C2H6Si)n
- Structure:
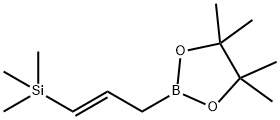
- Chemical Name:4,4,5, 5-tetramethyl-2-[(2E)-3-(trimethylsilyI) -2-propen- 1-yI]-1,3,2-Dioxaborolane
- CAS:79309-68-5
- MF:C12H25BO2Si
- Structure:
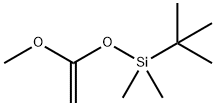
- Chemical Name:1-(tert-Butyldimethylsilyloxy)-1-methoxyethene
- CAS:77086-38-5
- MF:C9H20O2Si
- Structure:
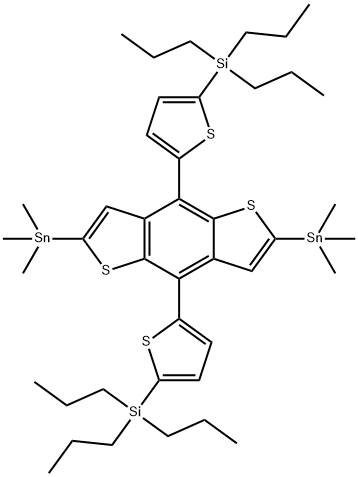
- Chemical Name:2,6-Bis(trimethylstannyl)-4,8-bis(5-(tripropylsilyl)thiophen-2-yl)benzo[1,2-b:4,5-b’]dithiophene
- CAS:2035466-88-5
- MF:C42H66S4Si2Sn2
- Structure:
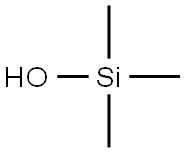
- Chemical Name:hydroxytrimethylsilane
- CAS:1066-40-6
- MF:C3H10OSi
- Structure:
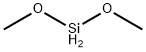
- Chemical Name:dimethoxysilane
- CAS:5314-52-3
- MF:C2H8O2Si
- Structure:
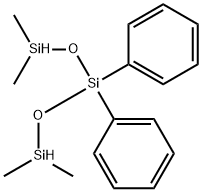
- Chemical Name:1,1,5,5-tetramethyl-3,3-diphenyltrisiloxane
- CAS:17875-55-7
- MF:C16H24O2Si3
- Structure:
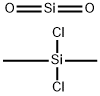
- Chemical Name:Silane, dichlorodimethyl-, reaction products with silica
- CAS:68611-44-9
- MF:C2H6Cl2O2Si2
- Structure:
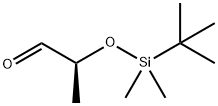
- Chemical Name:(S)-2-(tert-Butyldimethylsilyloxy)propanal
- CAS:87727-28-4
- MF:C9H20O2Si
- Structure:
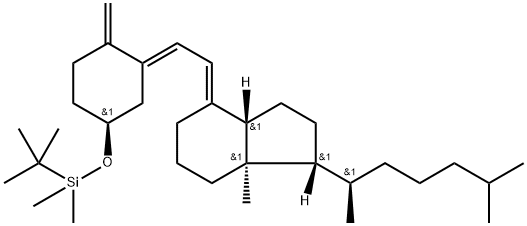
- Chemical Name:Silane, (1,1-diMethylethyl)diMethyl[[(3β,5E,7E)-9,10-secocholesta-5,7,10(19)-trien-3-yl]oxy]-
- CAS:87649-55-6
- MF:C33H58OSi
- Structure:
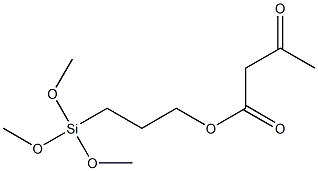
- Chemical Name:Butanoic acid, 3-oxo-, 3-(trimethoxysilyl)propyl ester
- CAS:121505-13-3
- MF:C10H20O6Si
- Chemical Name:Hydroxy silicone oil
- CAS:
- MF:(C2H6OSi)nC26H54O5Si2
- Structure:
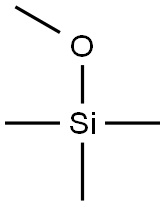
- Chemical Name:Silicone oil (high temperature)
- CAS:63148-58-3
- MF:C4H12OSi
- Structure:
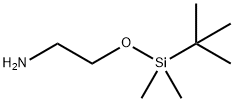
- Chemical Name:2-(tert-butyldiMethylsilyloxy)ethanaMine
- CAS:101711-55-1
- MF:C8H21NOSi
- Structure:
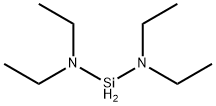
- Chemical Name:Bis(diethylamino)silane
- CAS:27804-64-4
- MF:C8H22N2Si
- Structure:
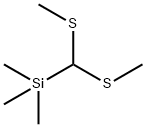
- Chemical Name:BIS(METHYLTHIO)(TRIMETHYLSILYL)METHANE
- CAS:37891-79-5
- MF:C6H16S2Si
- Structure:
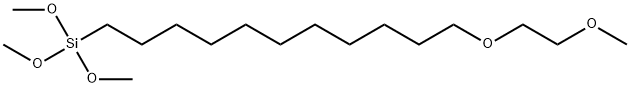
- Chemical Name:3,3-Dimethoxy-2,15,18-trioxane-3-silaundecane
- CAS:1384163-86-3
- MF:C17H38O5Si
- Structure:
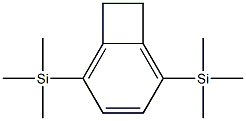
- Chemical Name:Silane, bicyclo[4.2.0]octa-1,3,5-triene-2,5-diylbis[trimethyl-
- CAS:132170-05-9
- MF:C14H24Si2
- Structure:
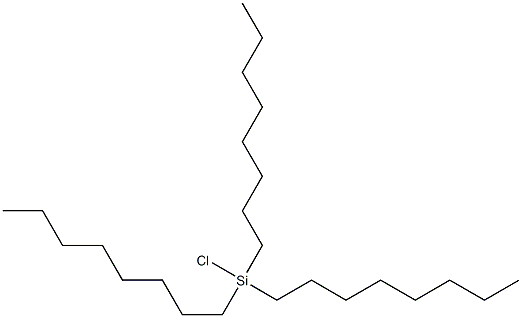
- Chemical Name:Silane, chlorotrioctyl-
- CAS:18768-14-4
- MF:C24H51ClSi
- Structure:
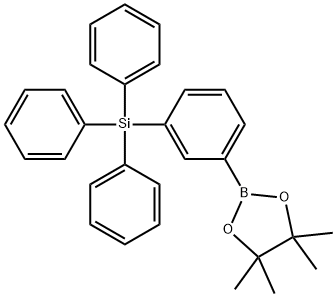
- Chemical Name:triphenyl[3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]silane
- CAS:1391041-75-0
- MF:C30H31BO2Si
- Structure:

- Chemical Name:Silicon tetrahydride
- CAS:7803-62-5
- MF:H4Si
- Structure:
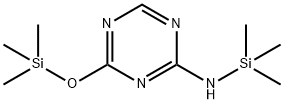
- Chemical Name:N-(Trimethylsilyl)-4-[(trimethylsilyl)oxy]-2-amine-1,3,5-triazin
- CAS:52523-35-0
- MF:C9H20N4OSi2
- Structure:
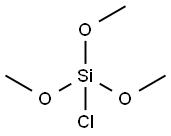
- Chemical Name:Trimethoxychlorosilane
- CAS:4668-00-2
- MF:C3H9ClO3Si
- Structure:
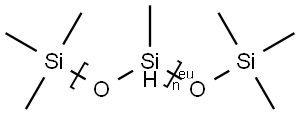
- Chemical Name:POLYMETHYLHYDROSILOXANE
- CAS:178873-19-3
- MF:((CH4OSi)nC6H18OSi2)x
- Chemical Name:CARBINOL (HYDROXYL) TERMINATED POLYDIMETHYLSILOXANE, 400-450 cSt
- CAS:68937-54-2
- MF:
- Structure:
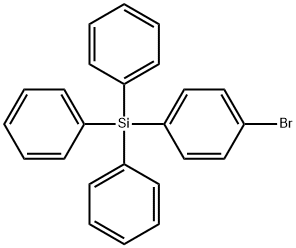
- Chemical Name:4-Bromotetraphenylsilane
- CAS:18737-40-1
- MF:C24H19BrSi
- Structure:
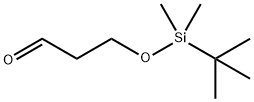
- Chemical Name:3-[(TERT-BUTYLDIMETHYLSILYL)OXY]-1-PROPANAL
- CAS:89922-82-7
- MF:C9H20O2Si
- Chemical Name:mPEG-Silane
- CAS:
- MF:
- Structure:
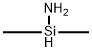
- Chemical Name:Poly(1,1-dimethylsilazane)
- CAS:89535-60-4
- MF:C2H9NSi
- Structure:
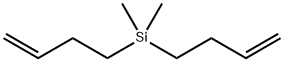
- Chemical Name:Silane,di-3-buten-1-yldimethyl-
- CAS:24171-45-7
- MF:C10H20Si
- Structure:
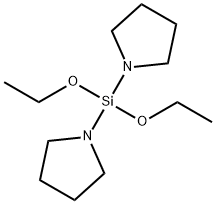
- Chemical Name:Diethoxy-Di(Pyrrolyl-1-Yl)Silane
- CAS:1353744-76-9
- MF:C12H26N2O2Si
- Structure:
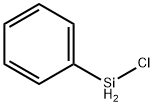
- Chemical Name:CHLOROPHENYLSILANE
- CAS:4206-75-1
- MF:C6H7ClSi
- Structure:
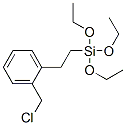
- Chemical Name:[2-[(chloromethyl)phenyl]ethyl]triethoxysilane
- CAS:10088-50-3
- MF:C15H25ClO3Si
- Structure:
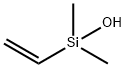
- Chemical Name:Silanol, ethenyldimethyl-
- CAS:5906-75-2
- MF:C4H10OSi
- Structure:
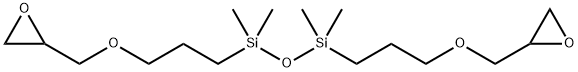
- Chemical Name:1,3-bis(3-glycidoxypropyl)tetramethyldisiloxane
- CAS:126-80-7
- MF:C16H34O5Si2
- Structure:
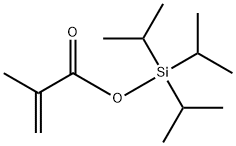
- Chemical Name:triisopropylsilyl Methacrylate
- CAS:134652-60-1
- MF:C13H26O2Si
- Structure:
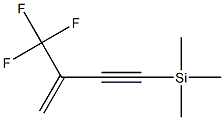
- Chemical Name:trimethyl[3-(trifluoromethyl)-3-buten-1-yn-1-yl]- Silane
- CAS:152327-70-3
- MF:C8H11F3Si
- Structure:
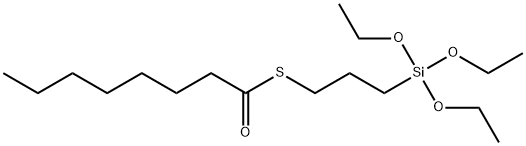
- Chemical Name:S-(Octanoyl)mercaptopropyltriethoxysilane
- CAS:220727-26-4
- MF:C17H36O4SSi
- Structure:
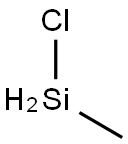
- Chemical Name:Chloromethyl silane
- CAS:993-00-0
- MF:CH5ClSi
- Chemical Name:Vinyl silicone oil
- CAS:
- MF: