Inorganic acid Esters
Inorganic acid ester is obtained through the reaction between alcohol and oxygen-containing organic acids or inorganic acid to generate esters (inorganic acid ester or organic acid esters) and water reaction. For example, sulfuric acid and ethanol, nitric acid and glycerol or pentaerythritol esters can have esterification reaction to generate important inorganic acids ester.
The compound obtained through the dehydration reaction between acid (inorganic and organic acids) and the alcohol is ester. It can be divided into inorganic acid esters (e.g., dimethyl sulfate, methyl hydrogen sulfate, glyceryltrinitrate, tributyl phosphate, etc.), and organic acid esters (e.g., ethyl acetate, vinyl acetate, and [alpha] methyl methacrylate ester, etc.) two categories. But the latter one is more important. In accordance with the intramolecular or intermolecular esterification of hydroxy acid (alcohol acid), it can also be divided into two kinds, lactone and lactide. Ester is generally neutral substance and can be subject to hydrolysis. Carboxylic acid ester can participate in a series of reactions including transesterification (alcoholysis), ammonolysis, reduction (hydrogenolysis) and the Grignard reaction.
Usually refers to a carboxylic acid ester with the general formula being RCOOR ', being the product with the hydrogen in the carboxyl group of carboxylic acid being replaced by hydrocarbon. The names of the esters are based on the names of corresponding carboxylic acid and alcohol or phenol such as "a certain acid ester." The cyclic ester is called lactone.
The chemical properties of ester are similar to acyl halide and acid anhydride. It is prone to have hydrolysis, alcoholysis and aminolysis reaction. The lower esters are aromatic volatile colorless liquid with the high-grade ester being solid. Esters are important solvents and synthetic chemicals with some kinds of ester itself being medicines. Depending on the type of acid, esters can be divided into inorganic and organic esters, such as hydrogen methyl sulfate (CH3OSO3H) for the former one and ethyl acetate CH3COOCH2CH3 for the later one; depending on the type of hydrocarbon, the ester can also be divided into fatty esters and aromatic esters and ring esters; ethyl acetate belongs to fatty esters; the phenyl acetate belongs to aromatic esters; while methyl furoate belongs to cyclic ester.
From the broad definition, the reaction between alcohols and acids for generating ester should be called as esterification. But usually it only refers to acid esterification and the esterification of alcohol is called as acylation. Esterification is reversible with the ester having hydrolysis with water to generate the original alcohol (or phenol) and acid. In general, the esterfication should be conducted in the presence of catalyst (hydrogen ion) and heating conditions, in order to shorten the time required for reaching equilibrium.
- Structure:
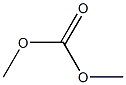
- Chemical Name:DimethylCarbonate
- CAS:
- MF:C3H6O3
- Chemical Name:STARCH PHOSPHATE
- CAS:
- MF:
- Structure:
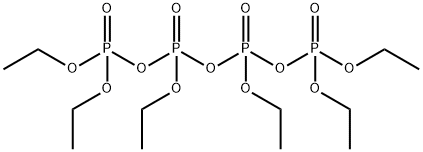
- Chemical Name:Hexaethyl tetraphosphate
- CAS:757-58-4
- MF:C12H30O13P4
- Structure:
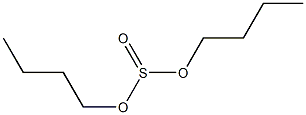
- Chemical Name:dibutyl sulfite
- CAS:
- MF:C8H18O3S
- Structure:
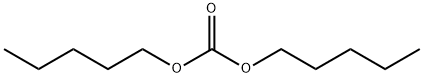
- Chemical Name:CARBONICACID,DIPENTYLESTER
- CAS:2050-94-4
- MF:C11H22O3
- Structure:
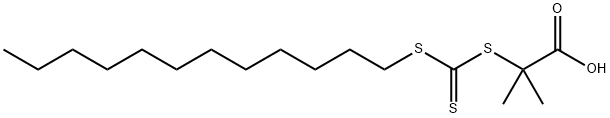
- Chemical Name:2-(DODECYLSULFANYLTHIOCARBONYLSULFANYL)-2-METHYLPROPIONIC ACID
- CAS:461642-78-4
- MF:C17H32O2S3
- Structure:
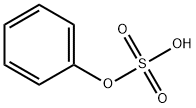
- Chemical Name:phenylsulfate
- CAS:937-34-8
- MF:C6H6O4S
- Structure:
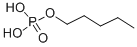
- Chemical Name:Pentyl dihydrogen phosphate
- CAS:12789-46-7
- MF:C5H13O4P
- Structure:
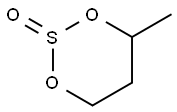
- Chemical Name:4-methyl-1,3,2-dioxathiane 2-oxide
- CAS:4426-51-1
- MF:C4H8O3S
- Structure:
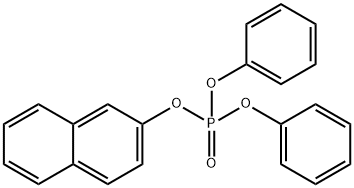
- Chemical Name:phosphoric acid,2-naphthalenyl diphenyl ester
- CAS:18872-49-6
- MF:C22H17O4P
- Structure:

- Chemical Name:Propyl butylsulfonate
- CAS:
- MF:C7H16O3S
- Structure:
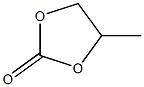
- Chemical Name:propylene carbonate
- CAS:
- MF:C4H6O3
- Chemical Name:2,3-Butylene carbonate
- CAS:
- MF:C5H8O3
- Structure:
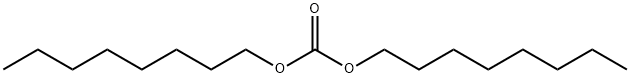
- Chemical Name:Dicaprylyl carbonate
- CAS:1680-31-5
- MF:C17H34O3
- Structure:
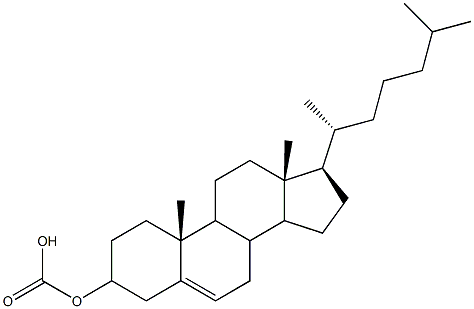
- Chemical Name:Cholesteryl carbonate
- CAS:
- MF:C28H46O3
- Structure:
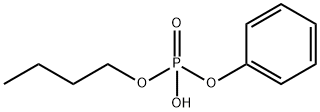
- Chemical Name:Phosphoric acid, monobutyl monophenyl ester
- CAS:46438-39-5
- MF:C10H15O4P
- Chemical Name:allyl diglycocarbonate
- CAS:
- MF:C12H14O7
- Structure:
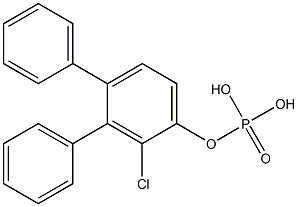
- Chemical Name:diphenyl-o-chlorophenyl phosphate
- CAS:
- MF:C18H14ClO4P
- Structure:

- Chemical Name:n-octyl nitrate
- CAS:
- MF:C8H17NO3
- Structure:
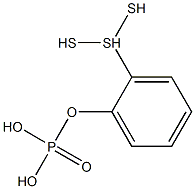
- Chemical Name:trithiophenyl phosphate
- CAS:
- MF:C6H9O4PS3
- Structure:
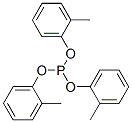
- Chemical Name:tris(methylphenyl) phosphite
- CAS:25586-42-9
- MF:C21H21O3P
- Chemical Name:1-(2-Pyridyla20)-2-Naphthol(Pan)
- CAS:
- MF:
- Structure:

- Chemical Name:Methyl Bisulfate
- CAS:
- MF:CH4O4S
- Structure:
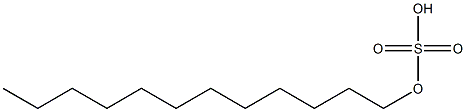
- Chemical Name:dodecyl sulfate
- CAS:
- MF:C12H26O4S
- Structure:
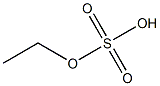
- Chemical Name:monoethyl sulfate
- CAS:
- MF:C2H6O4S
- Structure:
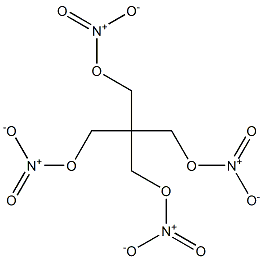
- Chemical Name:pentaerythitol tetranitrate
- CAS:
- MF:C5H8N4O12
- Structure:

- Chemical Name:decyl nitrate
- CAS:
- MF:C10H21NO3
- Structure:
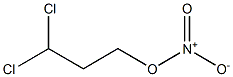
- Chemical Name:dichloropropyl nitrate
- CAS:
- MF:C3H5Cl2NO3
- Structure:
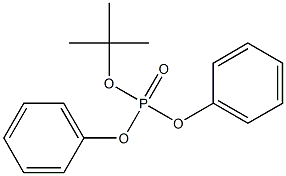
- Chemical Name:diphenyl tert-butyl phosphate
- CAS:
- MF:C16H19O4P
- Structure:
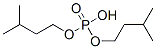
- Chemical Name:Di-iso-amyl phosphate
- CAS:3985-20-4
- MF:C10H23O4P
- Structure:
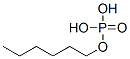
- Chemical Name:Phosphoric acid, hexyl ester
- CAS:68511-03-5
- MF:C6H15O4P
- Structure:

- Chemical Name:DIETHYLHEXYL CARBONATE
- CAS:
- MF:C11H22O3
- Structure:
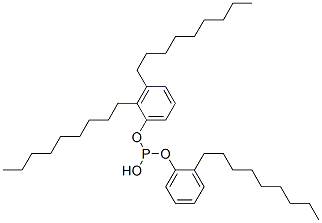
- Chemical Name:Phosphorous acid, dinonylphenyl nonylphenyl ester
- CAS:68186-33-4
- MF:C24H42O.xC15H24O.xH3O3P
- Structure:
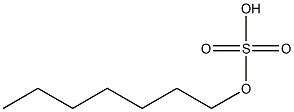
- Chemical Name:heptyl sulfate
- CAS:
- MF:C7H16O4S
- Structure:

- Chemical Name:n-octyl nitrite
- CAS:
- MF:C8H17NO2
- Structure:
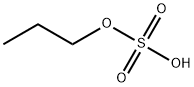
- Chemical Name:propyl hydrogen sulfate
- CAS:13425-84-8
- MF:C3H8O4S
- Structure:

- Chemical Name:1-decanol sulfate
- CAS:
- MF:C10H22O4S
- Chemical Name:Cellulose, dinitrate
- CAS:50935-18-7
- MF:
- Structure:
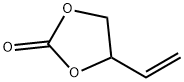
- Chemical Name:4427-96-7
- CAS:4427-96-7
- MF:
- Chemical Name:Poly(oxy-1,2-ethanediyl), .alpha.-sulfo-.omega.-hydroxy-, C6-10-alkyl ethers, ammonium salts
- CAS:68037-05-8
- MF:
- Structure:
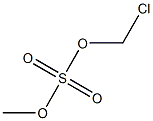
- Chemical Name:chloromethyl methyl sulfate
- CAS:
- MF:C2H5ClO4S
- Structure:

- Chemical Name:decyl nitrite
- CAS:
- MF:C10H21NO2
- Structure:
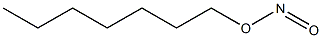
- Chemical Name:heptyl nitrite
- CAS:
- MF:C7H15NO2
- Chemical Name:mononitroglycerin
- CAS:
- MF:C3H5(OH)2ONO2
- Structure:
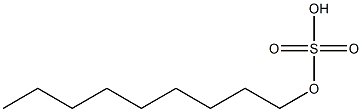
- Chemical Name:nonyl sulfate
- CAS:
- MF:C9H20O4S
- Structure:
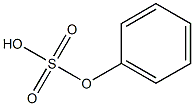
- Chemical Name:phenyl hydrogen sulfate
- CAS:
- MF:C6H6O4S
- Chemical Name:Isoamyl nitrate γ-Methylbutyl nitrate
- CAS:
- MF:C5H11NO3
- Structure:
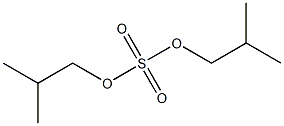
- Chemical Name:diisobutyl sulfate
- CAS:
- MF:C8H18O4S
- Structure:
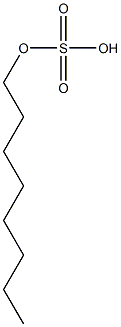
- Chemical Name:n-octyl sulfate
- CAS:
- MF:C8H18O4S
- Structure:
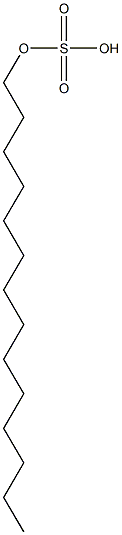
- Chemical Name:tetradecyl sulfate
- CAS:
- MF:C14H30O4S
- Structure:

- Chemical Name:Butyl orthosilicate
- CAS:
- MF:C4H12O4Si
- Chemical Name:π-Propyl nitrite
- CAS:
- MF:
- Structure:

- Chemical Name:isotridecyl dihydrogen phosphate
- CAS:50977-11-2
- MF:C13H29O4P
- Structure:
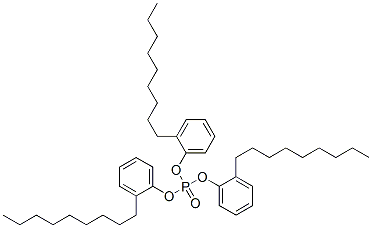
- Chemical Name:tris(nonylphenyl) phosphate
- CAS:26569-53-9
- MF:C45H69O4P
- Structure:
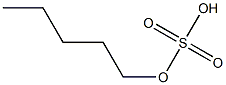
- Chemical Name:amyl hydrogen sulfate
- CAS:
- MF:C5H12O4S
- Structure:
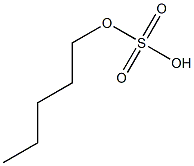
- Chemical Name:amyl sulfate
- CAS:
- MF:C5H12O4S
- Structure:
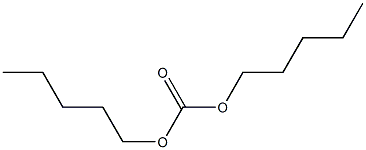
- Chemical Name:diamyl carbonate
- CAS:
- MF:C11H22O3
- Structure:
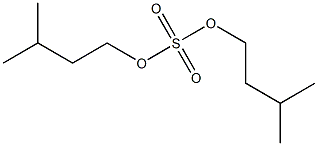
- Chemical Name:diisoamyl sulfate
- CAS:
- MF:C10H22O4S
- Structure:
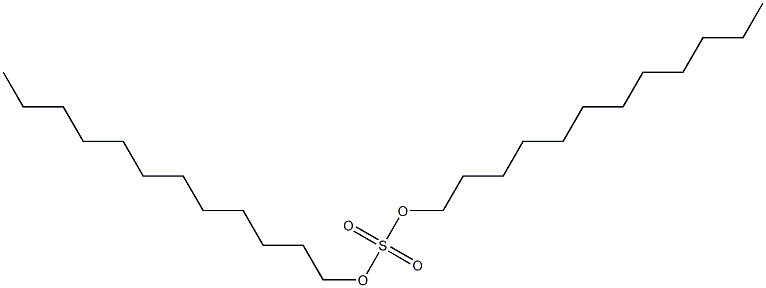
- Chemical Name:di-n-dodecyl sulfate
- CAS:
- MF:C24H50O4S
- Structure:
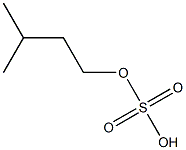
- Chemical Name:isoamyl sulfate
- CAS:
- MF:C5H12O4S
- Chemical Name:nitromannite
- CAS:
- MF:C6H8(NO3)6
- Structure:
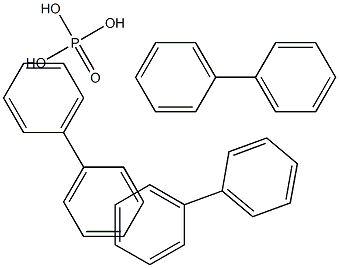
- Chemical Name:tri-p-biphenyl phosphate
- CAS:
- MF:C36H33O4P
- Structure:
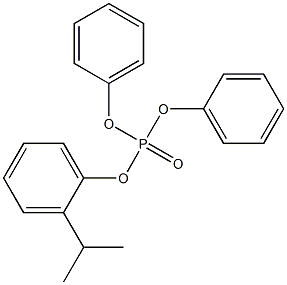
- Chemical Name:ISOPROPYLPHENYLDIPHENYL PHOSPHATE
- CAS:
- MF:C21H21O4P
- Structure:

- Chemical Name:Diethyl silicate
- CAS:
- MF:Si2Ti
- Structure:
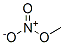
- Chemical Name:Methyl nitrate
- CAS:589-58-3
- MF:CH3NO3
- Structure:
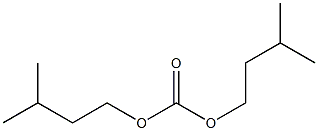
- Chemical Name:carbonic acid diisoamyl ester
- CAS:
- MF:C11H22O3
- Structure:
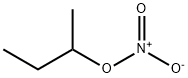
- Chemical Name:sec-butylnitrate
- CAS:924-52-7
- MF:C4H9NO3
- Structure:
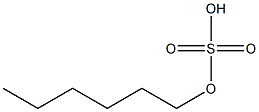
- Chemical Name:hexyl sulfate
- CAS:
- MF:C6H14O4S
- Structure:
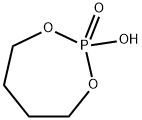
- Chemical Name:tetramethylene phosphate
- CAS:51374-71-1
- MF:C4H9O4P
- Structure:
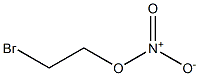
- Chemical Name:2-bromoethyl nitrate
- CAS:
- MF:C2H4BrNO3
- Structure:
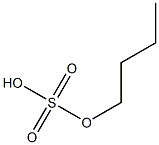
- Chemical Name:butyl hydrogen sulfate
- CAS:
- MF:C4H10O4S
- Structure:
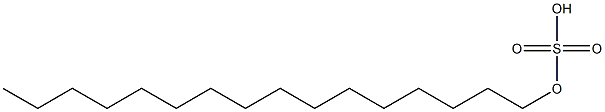
- Chemical Name:cetyl sulfate
- CAS:
- MF:C16H34O4S
- Structure:
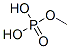
- Chemical Name:Phosphoric acid,methyl ester
- CAS:12789-45-6
- MF:CH4O.xH3O4P
- Structure:

- Chemical Name:Ethyl butyl carbonate
- CAS:
- MF:C7H14O3
- Chemical Name:SULFATED GLYCERYL OLEATE
- CAS:
- MF: