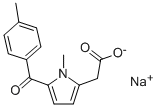
Tolmetin sodium
한글명:Tolmetin sodium
카스 번호35711-34-3
상품명:Tolmetin sodium
CBNumberCB6397353
분자식C15H14NNaO3
포뮬러 무게279.27
MOL 파일35711-34-3.mol
그림문자(GHS)
-
그림문자(GHS)

-
신호 어
Warning
-
유해·위험 문구
H302:삼키면 유해함
-
예방조치문구
P264:취급 후에는 손을 철저히 씻으시오.
P264:취급 후에는 손을 철저히 씻으시오.
P270:이 제품을 사용할 때에는 먹거나, 마시거나 흡연하지 마시오.
P301+P312:삼켜서 불편함을 느끼면 의료기관(의사)의 진찰을 받으시오.
P330:입을 씻어내시오.
P501:...에 내용물 / 용기를 폐기 하시오.
Tolmetin sodium 화학적 특성, 용도, 생산
-
용도
Tolmetin Sodium is a non-steroidal anti-inflammatory drug. -
Pharmacokinetics
Tolmetin sodium is rapidly and almost completely absorbed on oral administration, with peak plasma levels being attained within the first hour of administration. It has a relatively short plasma half-life of approximately 1 hour because of extensive first-pass metabolism, involving hydroxylation of the p-methyl group to the primary alcohol, which is subsequently oxidized to the dicarboxylic acid. -
Clinical Use
Tolmetin is synthesized straightforwardly from 1-methylpyrrole. It was introduced in the United States in 1976 and like other NSAIDs, inhibits prostaglandin biosynthesis. Tolmetin, however, also inhibits polymorph migration and decreases capillary permeability. Its anti-inflammatory activity, as measured in the carrageenan-induced rat paw edema and cotton pellet granuloma assays, is intermediate between those of phenylbutazone and indomethacin. -
부작용
The most frequently adverse reactions are those involving the GI tract (e.g., abdominal pain, discomfort, and nausea) but appear to be less than those observed with aspirin. The CNS effects (e.g., dizziness and drowsiness) also are observed. Few cases of overdosage have been reported, but in such cases, recommended treatment includes elimination of the drug from the GI tract by emesis or gastric lavage and elimination of the acidic drug from the circulatory system by enhancing alkalinization of the urine with sodium bicarbonate. -
신진 대사
This metabolite is inactive in standard in vivo anti-inflammatory assays. The free acid (pKa = 3.5) is highly bound to plasma proteins (99%), and excretion of tolmetin and its metabolites occurs primarily in the urine.Approximately 15 to 20% of an administered dose is excreted unchanged and 10% as the glucuronide conjugate of the parent drug. Conjugates of the dicarboxylic acid metabolite account for the majority of the remaining administered drug.
Tolmetin sodium 공급 업체
Global(50)Suppliers
-
CONIER CHEM AND PHARMA LIMITED
전화 +8618523575427
이메일 sales@conier.com
-
전화+86-0371-86658258<br/>+8613203830695
이메일 laboratory@coreychem.com
-
Hefei TNJ Chemical Industry Co.,Ltd.
전화+86-0551-65418671<br/>+8618949823763
이메일 sales@tnjchem.com
-
Dideu Industries Group Limited
전화+86-29-89586680<br/>+86-15129568250
이메일 1026@dideu.com
-
전화 +8618058761490
이메일 info@gihichemicals.com
-
전화 +8613564774135
이메일 zijue.cai@tsbiochem.com
-
Hangzhou Yuhao Chemical Technology Co., Ltd
전화0571-82693216
이메일 info@yuhaochemical.com
-
Beijing HuaMeiHuLiBiological Chemical
전화010-56205725
이메일 waley188@sohu.com
-
Shanghai Hekang Biotechnology Co., Ltd.
전화 18939837085
이메일 youchemicals@gmail.com
-
Beijing Solarbio Science & Tecnology Co., Ltd.
전화010-50973186<br/>4009686088
이메일 3193328036@qq.com
1of2