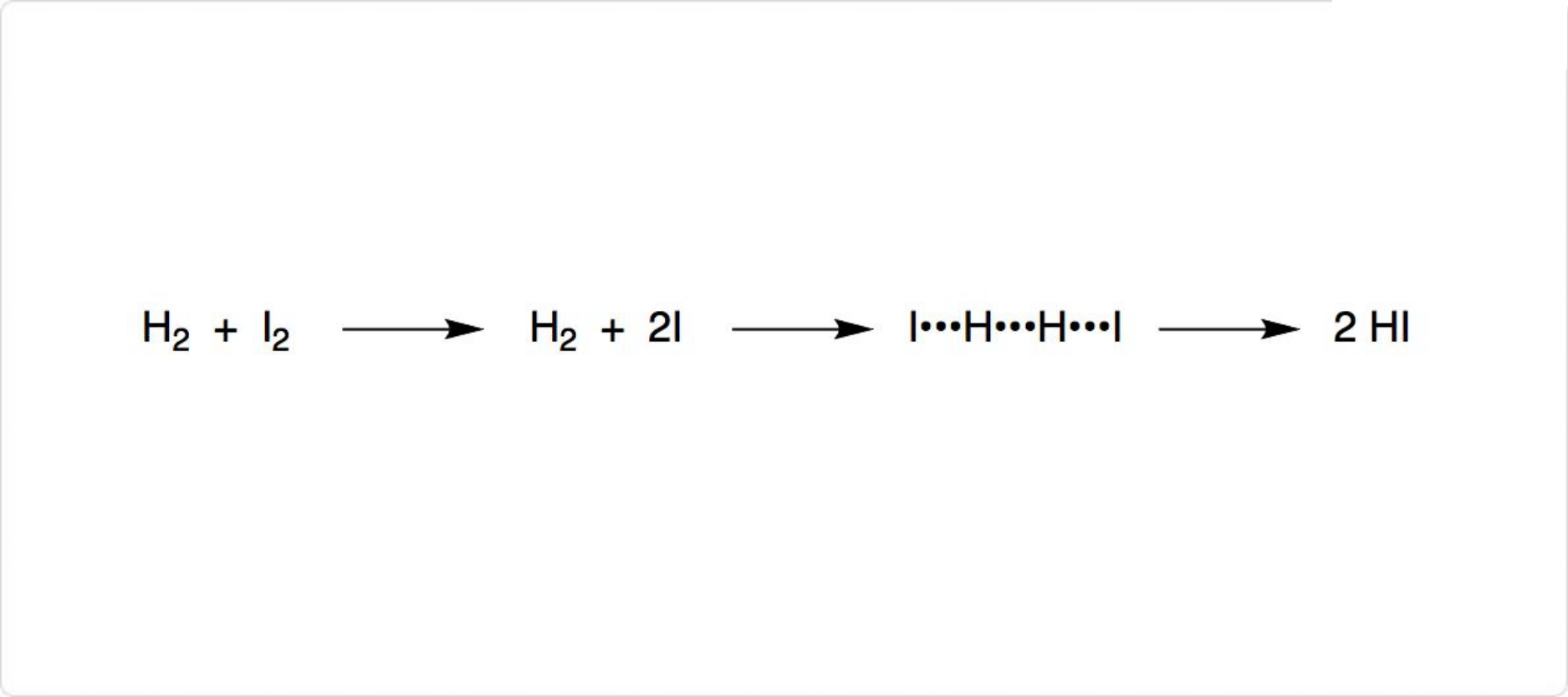-
外観
無色~褐色, 澄明の液体
-
性質
ヨウ化水素の融点は−50.8°C、沸点は−35.1°Cです。常温では無色で刺激臭のある気体です。還元力が強いため、空気中の酸素によって容易に酸化して、赤紫色のヨウ素を生じます。ヨウ化水素の酸化では、こげ茶色のHI3も生成され、ヨウ化水素の熟成溶液がこげ茶色に見える場合もよくあります。
水に非常によく溶け、塩化水素や臭化水素と同じく、水に対する溶解熱は非常に大きいです。イオン半径は大きいヨウ化物イオンと水素イオンの静電気力は小さく、電離しやすいため、水溶液は強い酸性を示し、pKaは−10です。
-
溶解性
水及びエタノールと混和する。
-
解説
ようかすいそ,無色,刺激性のある発煙性の気体で,液体は淡黄色を呈する。融点は-50.8℃,沸点-35.1℃,臨界温度150.8℃。気体の密度は5.66g/cm3(0℃,1気圧)で,空気の4.77倍。液体の密度は2.799g/cm3(-35.4℃)。気体におけるHI分子のH―I結合距離は1.609Å。双極子モーメントは0.38D(Dは双極子モーメントの単位デバイの略で,1D=3.336×10-30C・m)で,H-I結合のイオン性は5%。
株式会社平凡社 世界大百科事典 第2版について 情報
-
用途
汎用試薬。還元剤。
-
用途
医薬中間原料、試薬、殺虫剤
-
用途
分析用試薬,医薬原料(化学工業日報社)
-
構造
ヨウ素から構成されるハロゲン化水素の1種です。分子量は 127.90g/molであり、-47°Cでの密度は2.85g/mLです。化学式はHIで表されます。
水素とヨウ素の電気陰性度にはほとんど差がないため、分子の極性は小さいです。水素原子とヨウ素原子の距離は160.9pmです。
-
合成法
工業的に、ヨウ素とヒドラジンの反応によって窒素ガスが発生し、ヨウ化水素が得られます。水中での反応の場合には、ヨウ化水素を蒸留する必要があります。
ヨウ化物にを加えて加熱しても、ヨウ化水素を合成可能です。ヨウ素水溶液に硫化水素ガスを吹き込んで、ヨウ化水素酸と硫黄を生成する方法もあります。
実験室では、水とヨウ素の混合物にを加えて、PI3の加水分解によって生成可能です。この反応では、I2とリンの反応によりPI3が生じ、水と反応するとヨウ化水素と亜リン酸が生成されます。
-
説明
‘Iodine’ is derived from iodes, a Greek word meaning violet. It is
a member of the halide family and hydrogen iodide is
considered a strong acid.
-
化学的特性
Hydrogen iodide is a colourless to yellow/brown with an acrid odour non-flammable gas. Hydrogen iodide is incompatible with water and other halides. Hydrogen iodide, upon contact with moisture in air, releases dense vapours. Hydrogen iodide reacts with water to form corrosive acids and reacts violently with alkalis. Most metals corrode rapidly on contact with wet hydrogen iodide, and prolonged exposure of hydrogen iodide to fire or intense heat has been reported to cause the container to rupture and rocket.
-
物理的性質
This is a strong acid, made by dissolving HI gas in
water. However, hydrogen iodide and hydroiodic acid
differ in that the former is a gas under standard conditions
whereas the other is an aqueous solution of said
gas. They are noninterconvertible. That is, once the
acid is formed with water, it cannot be recovered like
HCl or HBr. Hydroiodic acid is used in organic and inorganic
synthesis as one of the primary sources of iodine
and as a reducing agent.
With moist air, HI gas gives a mist (or fumes) of
hydroiodic acid. It is exceptionally soluble in water.
One liter of water will dissolve 425 L of HI, the final
solution containing only four water molecules per molecule
of HI. As stated, although chemically related,
hydroiodic acid is not pure HI but a mixture containing it. Commercial “concentrated” hydroiodic acid usually
contains 90–98% HI by mass.
-
使用
Hydriodic acid is used in the manufactureof iodides, as a reducing agent, and indisinfectants and pharmaceuticals.
-
定義
hydrogen iodide: A colourless gas,HI; m.p. –51°C; b.p. –35.38°C. It canbe made by direct combination ofthe elements using a platinum catalyst.It is a strong acid dissociating extensivelyin solution (hydroiodic acid or hydriodic acid). It is also a reducingagent.
-
製造方法
Hydrogen iodide is prepared by direct combination of hydrogen and iodinevapor in the presence of platinum catalyst:
H2 + I2 → 2HI
The compound is produced in commercial scale by reaction of iodine withhydrazine or hydrogen sulfide:
2I2 + N2H4 → 4HI + N2
I2 + H2S → 2HI + S
Hydriodic acid may be prepared by dissolving hydrogen iodide gas in water.The acid also may be obtained by electrolysis of iodine solution or by passinghydrogen sulfide into a suspension of iodine in water and boiling to expelexcess sulfide. After boiling, the precipitated sulfur is removed by filtrationthrough fritted glass plate or glass wool.
Hydriodic acid in small quantities may be prepared by adding water care-fully to a solid mixture of red phosphorus and iodine.
Technical grade hydriodic acid is a 47% HI solution and usually has abrown color due to the presence of free iodine, produced by air oxidation of HI.Hydriodic acid should be stored in the dark to prevent photochemical decom-position, and free from air to prevent oxidation. The addition of 1.5%hypophosphorus acid (H3PO2) prevents oxidative decomposition.
Hydriodic acid also is commercially sold at 57% (azeotropic concentration)and 10% aqueous solutions.
-
一般的な説明
A colorless to yellow liquid with a pungent odor. Consists of a solution of hydrogen iodide in water. Fumes irritate the eyes and mucous membranes. Corrosive to metals and to tissue.
It is prepared by the reaction of iodine and hydrosulfuric acid or by the reaction of phosphorus plus iodine plus water followed by distillation. Concentrated hydroiodic acid reacts with the oxygen of the air to form free iodine, which gives a brownish color to the solution. It also gives an idea of the reducing nature of this acid. It is an important reagent in organic chemistry and is used commercially in the preparation of iodides.
-
空気と水の反応
Soluble in water with release of heat.
-
反応プロフィール
HYDROIODIC ACID reacts exothermically with organic bases (amines, amides) and inorganic bases (oxides and hydroxides of metals). Reacts exothermically with carbonates (including limestone and building materials containing limestone) and hydrogen carbonates to generate carbon dioxide. Reacts with sulfides, carbides, borides, and phosphides to generate toxic or flammable gases. Reacts with many metals (including aluminum, zinc, calcium, magnesium, iron, tin and all of the alkali metals) to generate flammable hydrogen gas. Reacts violently with acetic anhydride, 2-aminoethanol, ammonium hydroxide, calcium phosphide, chlorosulfonic acid, 1,1-difluoroethylene, ethylenediamine, ethyleneimine, oleum, perchloric acid, b-propiolactone, propylene oxide, silver perchlorate/carbon tetrachloride mixture, sodium hydroxide, uranium(IV) phosphide, vinyl acetate, calcium carbide, rubidium carbide, cesium acetylide, rubidium acetylide, magnesium boride, mercury(II) sulfate [Lewis]. Mixtures with concentrated sulfuric acid can evolve toxic hydrogen iodide gas at a dangerous rate. Decomposes at high temperatures to emit toxic products. Reacts with fluorine, dinitrogen trioxide, nitrogen dioxide/dinitrogen tetraoxide, and fuming nitric acid.
-
危険性
Strong irritant. Poison.
-
健康ハザード
Hydriodic acid is a corrosive liquid thatcan produce burns on contact with the skin.Contact of acid with the eyes can causesevere irritation. The gas, hydrogen iodide, isa strong irritant to the eyes, skin, and mucousmembranes. No exposure limit has been setfor this gas.
-
火災危険
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
-
化学性质
色で刺激臭の気体,融点?50.77℃,沸点?35.55℃
-
化学反応
ヨウ素と水素の反応
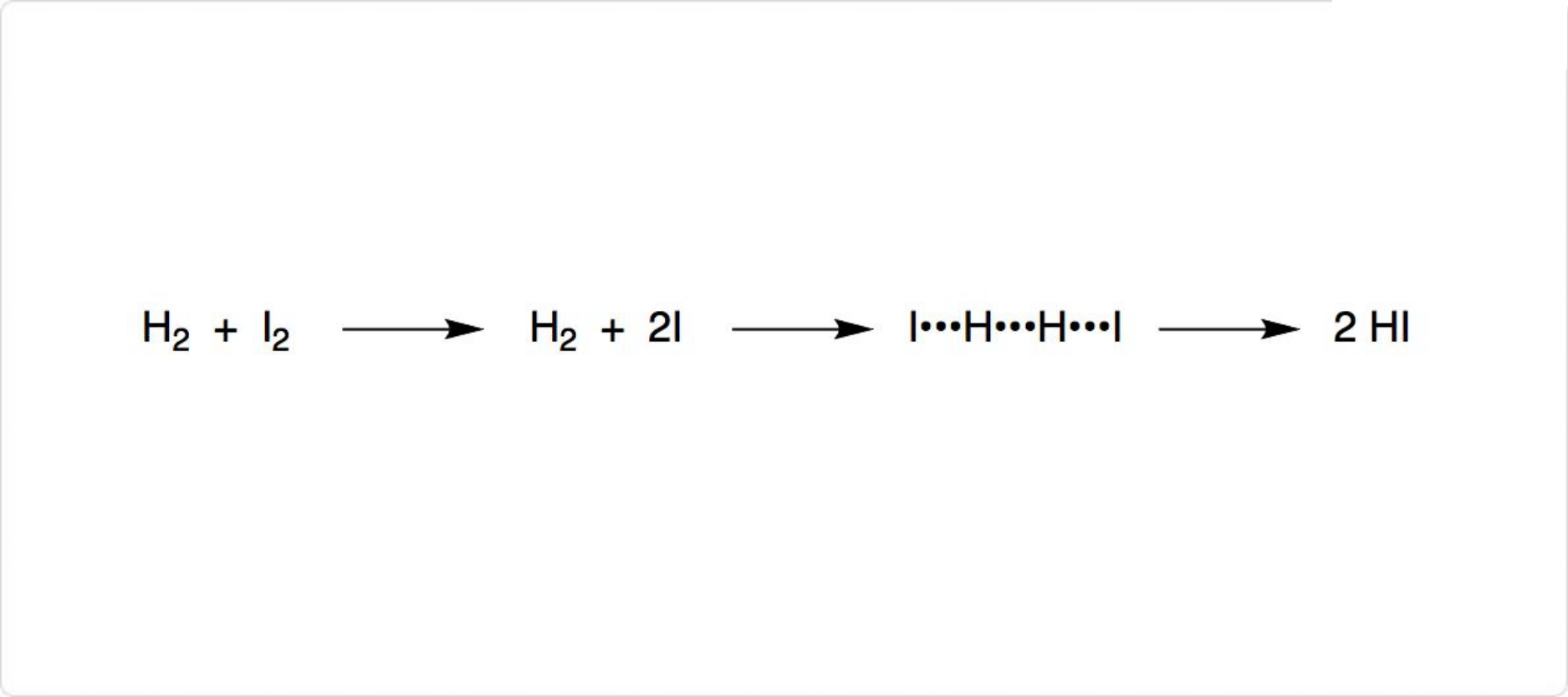
図1. ヨウ素と水素の反応
水素とヨウ素を組み合わせるだけでも、ヨウ化水素を合成可能です。通常この方法は、高純度のヨウ化水素を得るために使用されています。
H2とI2の反応では、まずI2が2つのヨウ素原子に解離して、それぞれがH2の側面に結合し、H-H結合を切断すると考えられています。I2の解離エネルギーである578nmに近い波長の光を照射すると、反応速度が著しく増加したためです。
ヨウ化水素によるSN2反応
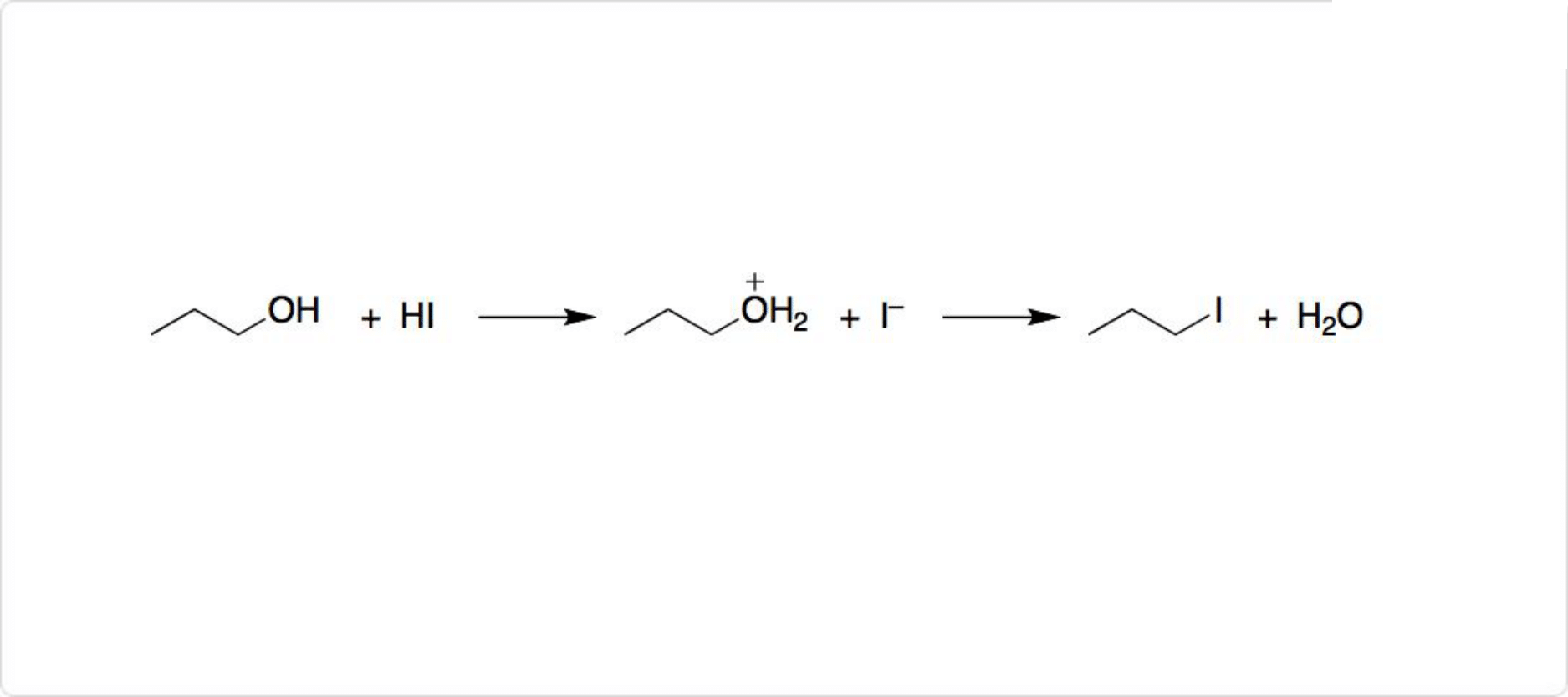
図2. ヨウ化水素のSN2反応
HBrやHClと同様に、HIはアルケンに付加します。有機化学でHIは、第一級アルコールをヨウ化アルキルに変換するために利用可能です。この反応はSN2置換であり、活性化された水酸基がヨウ化物イオンと交換されます。
ヨウ化水素によるSN1反応

図3. ヨウ化水素のSN1反応
臭化物や塩化物より優れた求核剤であるヨウ化物イオンは、加熱しなくても反応が起こりやすいです。二級アルコールや三級アルコールでは、SN1置換によって反応が進行します。
HIはエーテルをヨウ化アルキルとアルコールに切断できます。化学的に安定で不活性なエーテルを、反応性が高い化合物に変換できるため、重要な反応です。例えば、ジエチルエーテルをエタノールとヨードエタンに分解できます。
-
使用用途
ヨウ化水素は、同じハロゲン化水素である塩化水素やと比べて不安定で、酸化されやすいです。そのため、強い還元剤として利用されます。また、塩化物、臭化物、金属酸化物などと反応してヨウ化物を生成するため、無機ヨウ化物の製造に利用可能です。
さらに、ITO (インジウムスズ酸化物) のドライエッチング剤としても知られています。エッチングとは、物質の腐食作用を利用して、ICの回路を形成する工程のことです。
-
合成方法
ヨウ化物に濃リン酸溶液を加えて加熱する
-
純化方法
Iodine can be removed from aqueous HI, probably as the amine hydrogen triiodide, by three successive extractions using a 4% solution of Amberlite LA-2 (a long-chain aliphatic amine) in CCl4, toluene or pet ether (10mL per 100mL of acid). [Davidson & Jameson Chem Ind (London) 1686 1963.] Extraction with tributyl phosphate in CHCl3 or other organic solvents is also suitable. Alternatively, a De-acidite FF anion-exchange resin column in the OH--form using 2M NaOH, then into its I--form by passing dilute KI solution through, can be used. Passage of an HI solution under CO2 through such a column removes polyiodide. The column can be regenerated with NaOH. [Irving & Wilson Chem Ind (London) 653 1964]. The earlier method was to reflux with red phosphorus and distil in a stream of N2. The colourless product is stored in ampoules in the dark [Bradbury J Am Chem Soc 74 2709 1952, Heisig & Frykholm Inorg Synth I 157 1939]. It fumes in moist air. HARMFUL VAPOURS.
-
参考文献
A. Trummal, et al., J. Phys. Chem. A, 120, 3663 (2016), DOI: 10.1021/acs.jpca.6b02253.