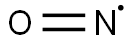-
性質
一酸化窒素は,高温における窒素と酸素の直接反応,白金触媒によるアンモニアの酸化などで得られる.自然界では,主として雷により生じる.実験室では,銅片と硫酸の反応で得られる.室温で無色の気体.融点-163.6 ℃,沸点-151.8 ℃.液体,固体ともに青色.双極子モーメント0.16 D.N-O0.114 nm.不対電子1個をもち,常磁性でほかの遊離基とよく反応する.この性質はポリマー表面の遊離基の検出に利用される.空気に触れると二酸化窒素になる.ハロゲンと反応してハロゲン化ニトロシルNOXをつくる.光化学スモッグや酸性雨の成因,オゾン層破壊の原因ともなり,大気汚染で問題となる窒素酸化物(NOx)の一つである.
-
解説
NO (mw30.01).酸化窒素ともいう.反応性の高い気体で,酸素と反応してNO2を生成する.動物細胞でアルギニンから生成し,平滑筋を弛緩させる作用,血小板凝集作用,好中球の活性化,血管拡張,線溶などの生理作用がある.グアニル酸シクラーゼを活性化して,cGMPを上昇させる.哺乳動物に広く分布する.
朝倉書店 栄養・生化学辞典について 情報
-
用途
シリコンの酸化膜形成用
-
用途
硝酸の製造中間体,レーヨンの漂白剤,半導体の製造などに用いられる.なお,一酸化窒素は細胞内でのシグナル伝達物質として重要な働きをする。
-
合成
オストワルト法による一酸化窒素の合成:白金触媒を用いて、900℃程度にアンモニアを熱すると、一酸化窒素が生成します。オストワルト法 (英: Ostwald process) と呼ばれ、一酸化窒素の一般的な生産方法です。
現在は白金にロジウムを10%程度加えた金網状の触媒が使用されます。温度が800°C、接触時間が0.001秒の条件で、白金-ロジウム触媒によって一酸化窒素が生成し、収率は95〜98%です。
-
効能
肺血管拡張薬
-
商品名
アイノフロー (エア・ウォーター,住友精化)
-
説明
near room temperature (its liquid density at 20°C is 1.45 g/cm3).
Nitrogen monoxide (NO) is commonly called nitric oxide,Nitric oxide is colorless and has a sharp sweet odor;Nitric oxide is nonfl ammable, toxic gases.Nitric oxide is a free radical that quickly reacts in air to produce nitrogen dioxide.It is also an
important biological messenger and transmitter.
-
化学的特性
Nitric oxide,NO, also known as nitrogen oxide and nitrogen monoxide, is a colorless gas that will react with oxygen at room temperature to form nitrogen dioxide, N202, a reddish-brown gas.It is soluble in water and alcohol and is used primarily to form other compounds.
-
物理的性質
Colorless gas; paramagnetic; density 1.3402 g/L; slightly heavier than air, air density 1.04 (air=1); liquefies at -151.8°C to a blue liquid; the refractive index of the liquid 1.330 at -90°C; the density of the liquid 1.269 g/mL at -150.2°C; solidifies at -163.6°C to a bluish-white snow-like solid; critical temperature -94°C; critical pressure 65 atm; slightly soluble in water, 4.6 mL gas dissolves in 100 mL water at 20°C while 7.34 mL and 2.37 mL dissolve in the same volume of water at 0 and 60°C, respectively; more soluble in alcohol than water; soluble in carbon disulfide, and in ferrous sulfate solution (reacts).
-
来歴
Nitric oxide was prepared in 1772 by Joseph Priestley
(1733–1804) and described in his volumes Experiments and Observations of Different Kinds
of Air published between 1774 and 1786. Priestley called nitric oxide nitrous air, nitrogen
dioxide nitrous acid vapor, and nitrous oxide phlogisticated nitrous air, but also referred to the
latter as diminished nitrous air. He observed the change of clear nitric oxide to red nitrogen
dioxide. Priestley prepared nitric oxide by reacting nitric acid with a metal such as copper:
3Cu(s) + 8HNO3(aq) → 2NO(g) + 3Cu(NO3)2(aq) + 4H2O(l).
-
使用
Nitric oxide is used as an intermediate in themanufacture of nitric acid, in the preparationof metal nitrosyls, in bleaching of rayon,and in incandescent lamps. It is produced byheating air at high temperatures.
-
定義
ChEBI: Nitric Oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom.
-
調製方法
Nitric oxide is commercially produced by the catalytic oxidation of ammonia using aplatinum catalyst: 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g).
-
一般的な説明
A colorless gas. Noncombustible but accelerates the burning of combustible material. Vapors heavier than air. Very toxic by inhalation and skin absorption. Heating the containers may cause them to rupture violently and rocket.
Nitric oxide was discovered by Van Helmont in 1620. It occurs in the exhaust gases from automobiles along with other oxides of nitrogen, at trace concentrations. It also is found in minute quantities in the upper atmosphere, resulting from the oxidation of nitrogen in the presence of ionizing radiation or by electric discharge. Nitric oxide is the most stable oxide of nitrogen. It is used as an intermediate or as a starting reactant in the production of many nitrogen compounds, including nitrogen dioxide, nitric acid and nitrosyl chloride.
-
空気と水の反応
Combines very rapidly with oxygen in the air to form nitrogen dioxide. Nitrogen dioxide reacts with water to form nitric acid and NITRIC OXIDE, reacts with alkalis to form nitrates and nitrites [Merck 11th ed. 1989].
-
反応プロフィール
NITRIC OXIDE can serve as both an oxidizing agent and as a reducing agent. Sustains the combustion of powdered aluminum [Mellor 5:209-212. 1946-47]. Enflames or explodes when mixed with vapors of carbon disulfide [Mellor 8, Supp. 2:232. 1967]. Reacts vigorously with sodium monoxide above 100°C [Mellor 2, Supp. 2:629. 1961]. Reacts on contact with oxygen at room temperature to form brown gaseous nitrogen dioxide. Reacts with alkalis to form nitrates and nitrites [Merck 11th ed. 1989]. The liquid is very sensitive to detonation in the presence of water.
-
危険性
Supports combustion. Toxic by inhalation,
strong irritant to skin and mucous membranes.
Hypoxia/cyanosis, nitrosyl-hemoglobin formation,
and upper respiratory tract irritant.
-
健康ハザード
Can cause death or permanent injury after a very short exposure to small quantities. Irritant of eyes, nose, throat; can cause unconsciousness. NITRIC OXIDE forms acids in the respiratory system which are irritating and cause congestion in the lungs. Concentrations of 60-150 ppm cause immediate irritation of the nose and throat with coughing and burning in the throat and chest. 6-24 hours after exposure, labored breathing and unconsciousness may result. Concentrations of 100-150 ppm are dangerous for short exposure of 30-60 minutes. Concentrations of 200-700 ppm may be fatal after very short exposure.
-
火災危険
Noncombustible gas; burns with fuels,
hydrocarbons, or when heated with hydrogen.
Nitric oxide reacts violently with carbon
disulfide vapors, producing green luminous
flame; with fluorine, it produces a pale yel low flame. It explodes when mixed with
ozone, chlorine monoxide, or a nitrogen tri halide. Reactions with many pyrophoric met als produce incandescence. Reaction with
amorphous boron produces brilliant flashes.
-
使用用途
一酸化窒素は、生体内で作られ続けています。生体内で生成した一酸化窒素は、動脈の内壁と外壁の間に位置する筋肉の「平滑筋」へ運ばれて、平滑筋の柔軟性を高め、動脈硬化を予防します。血管の柔軟性が保たれることで、血管に脂肪分が付着し、血流の悪化を防げます。
体内で作られる一酸化窒素の量は、加齢とともに減少します。適度な運動やアルギニンの摂取により、一酸化窒素の生成量を増やすことも可能です。
その一方で、一酸化窒素を使用して、ポリマーの表面のラジカルを検出できます。具体的には、X線光電子分光によって、一酸化窒素がラジカルを消去する際に生じる窒素を検出します。
-
化学反应
一酸化窒素は遷移金属と反応すると、金属ニトロシル (英: metal nitrosyl) と呼ばれる錯体が得られます。一般的にはM-NOと結合し、疑ハロゲン化物 (英: pseudohalide) 配位子として機能します。M-NOを持つ錯体のM-N-Oの結合角は、120°〜140°です。
-
化学性质
酸化窒素と余剰の残存酸素が反応して二酸化窒素を生成
-
危害
一酸化窒素による環境への影響:一酸化窒素は、大気汚染で問題になっている窒素酸化物 (NOx) の1つです。窒素酸化物は大気中の水蒸気と反応して硝酸になるため、酸性雨の原因になります。大気汚染防止法で窒素酸化物は、火力発電所、自動車、航空機、船舶などの排出源に対し、排出規制が実施されています。
高温で窒素と酸素から一酸化窒素が生じるため、自然界では雷や山火事が原因で一酸化窒素が生成しますが、人為的理由も多いです。具体的には、石油ストーブ、ガスコンロ、暖炉などで、排出規制対象にはなっていません。
大気に放出された一酸化窒素は二酸化窒素に酸化されますが、二酸化窒素は紫外線によって一酸化窒素と原子状酸素になります。原子状酸素はオゾンのような酸化物質を生成し、酸化物質の存在下では一酸化窒素の酸化が加速します。すなわち、連鎖的に反応が進行して、光化学オキシダントを生成するため、これが光化学スモッグを引き起こす原因です。
-
材料の用途
Nitric oxide is noncorrosive, and most common
structural materials may be used. However, in
the presence of moisture and oxygen, corrosive
conditions will develop as a result of the formation
of nitric and nitrous acids. Prior to use,
systems to contain nitric oxide must first be
purged with an inert gas. Where air contamination
cannot be eliminated, stainless steel should
be used.
-
職業ばく露
Nitric oxide is used in the manufacture
of nitric acid; it is also used in the bleaching of rayon;
it is a raw material for nitrosyl halide preparation.
-
環境運命予測
Nitric oxide is converted spontaneously in the air to nitrogen
dioxide; hence, some of the latter gas is present whenever nitric
oxide is found in air (at concentrations below 50 ppm). Nitric
oxide is a contributor to photochemical air pollution.
-
貯蔵
Nitric oxide should only be used in
well-ventilated areas. Valve protection caps and
valve outlet threaded plugs must remain in place
unless the container is secured and the valve
outlet piped to the point of use. Do not drag,
slide, or roll cylinders. Use a suitable hand truck
to move cylinders. Use a pressure reducing
regulator when connecting a cylinder to lower
pressure (1000 psig or 6900 kPa) piping systems.
Do not heat a cylinder of nitric oxide by
any means to increase the discharge rate from
the cylinder. Use a check valve or trap in the
discharge line to prevent hazardous reverse flow
into the cylinder.
-
合成方法
アンモニアの接触酸化
-
輸送方法
UN1660/124 Nitric oxide, compressed, Hazard
Class: 2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer,
8-Corrosive material, Inhalation Hazard Zone A. Cylinders
must be transported in a secure upright position, in a wellventilated
truck. Protect cylinder and labels from physical
damage. The owner of the compressed gas cylinder is the
only entity allowed by federal law (49CFR) to transport
and refill them. It is a violation of transportation regulations
to refill compressed gas cylinders without the express
written permission of the owner.
-
純化方法
Bubble the gas through 10M NaOH which removes NO2. It can also be freed from NO2 by passage through a column of Ascarite followed by a column of silica gel held at -197oK. The gas is dried with solid NaOH pellets or by passing through silica gel cooled at -78o, followed by fractional distillation from a liquid N2 trap. This purification does not eliminate nitrous oxide. Other gas scrubbers sometimes used include one containing conc H2SO4 and another containing mercury. It is freed from traces of N2 by the freeze and thaw method. [Blanchard Inorg Synth II 126 1946, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 485-487 1963.] TOXIC.
-
不和合性
A strong oxidizer but may also act as a
reducing agent. Explosive reaction with nitrogen
trichloride, ozone, carbon disulfide; pentacarbonyl iron;
chlorine monoxide. Incompatible with halogens, combustibles,
metals, oil, alcohols, chlorinated hydrocarbons (e.g.,
trichloroethylene), reducing agents (such as NH3), oxygen,
fluorine, metals. Reacts with water to form nitric acid.
Rapidly converted in air to nitrogen dioxide. Combines
very rapidly with oxygen in the air to form nitrogen dioxide.
Nitrogen dioxide reacts with water to form nitric acid
and nitric oxide, reacts with alkalis to form nitrates and
nitrites.
-
廃棄物の処理
Return refillable compressed
gas cylinders to supplier. Incineration with added hydrocarbon
fuel, controlled so as to produce elemental nitrogen,
CO2, and water. Consult with environmental regulatory
agencies for guidance on acceptable disposal practices.
Generators of waste containing this contaminant (≥100 kg/
mo) must conform with EPA regulations governing storage,
transportation, treatment, and waste disposal.
-
参考文献
1.https://en.wikipedia.org/wiki/Nitric_oxide
2.https://www.drugs.com/mtm/nitric-oxide-inhalation-gas.html
3.https://www.britannica.com/science/nitric-oxide
4.http://pediatrics.aappublications.org/content/106/2/344
5.http://www.mensfitness.com/nutrition/supplements/supplement-guide-nitric-oxide
6.https://www.chemicalbook.com/productchemicalpropertiescb5433122.htm