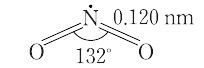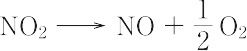-
性質
NO2(46.01).一酸化窒素と酸素を混合すると生成する褐色の気体.二量体である四酸化二窒素と平衡状態にあり,固体はほとんど純粋なN2O4.液体も沸点21.1 ℃ で約1% のNO2を含むだけであるが,気体は温度の上昇とともにNO2が増し,27 ℃,1 atm で27%,150 ℃ ではほとんど純粋なNO2となる.構造は図に示すような折れ線形で,非局在の不対電子1個をもち常磁性を示す.酸化力が強く,炭素,硫黄,リンなどはこのなかで燃える.水に作用させると硝酸と亜硝酸を生じる.600 ℃ 以上の高温で,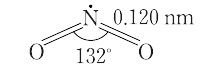 "
"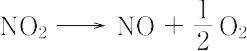 "のように分解する.硝酸の製造,硝化剤,酸化剤,アクリレートの重合禁止剤として用いられる.毒性が強く,吸入すると肺がおかされ,200 ppm 程度でも死に至るおそれがある.[CAS 10102-44-0][別用語参照]大気汚染,窒素酸化物
"のように分解する.硝酸の製造,硝化剤,酸化剤,アクリレートの重合禁止剤として用いられる.毒性が強く,吸入すると肺がおかされ,200 ppm 程度でも死に至るおそれがある.[CAS 10102-44-0][別用語参照]大気汚染,窒素酸化物
森北出版「化学辞典(第2版)
-
反応性
由一氧化氮和氧气混合产生的棕色气体。它与二聚体四氧化二氮平衡,固体几乎是纯的N 2 O 4。液体在沸点为 21.1°C 时也仅含有约 1% 的 NO 2 ,但气体会随着温度的升高而增加 NO 2并在27°C、1 atm和 27% 以及 150°C 时变成几乎纯的 NO 2 。如图所示,该结构呈线性弯曲一个离域不成对电子,并显示出顺磁性。氧化力强,碳、硫、磷等在其中燃烧。当作用于水时,它会产生硝酸和亚硝酸盐。在600°C或更高的高温下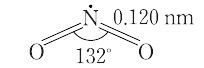
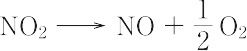
它像这样分解。用作硝酸生产的阻聚剂、硝化剂、氧化剂和丙烯酸酯。它具有剧毒,如果吸入,在 200 ppm 左右可能会损害肺部甚至死亡。
-
反応
二酸化窒素と四酸化二窒素は、平衡状態です。ルシャトリエの原理より平衡は、高温ほど二酸化窒素側へ移動します。液体窒素を用いて急速に冷やした場合には、二酸化窒素が固体として生成しますが、固体中には四酸化二窒素が存在しています。
また、水との反応で硝酸や亜硝酸が生じ、この反応が酸性雨の原因です。さらに、二酸化窒素と二酸化硫黄が反応すると、一酸化窒素と三酸化硫黄が得られます。
-
解説
二酸化窒素とは、窒素の酸化物で刺激臭のある気体です。
物質が高温で燃えるときに発生する (NO) が、大気中で酸化すると、二酸化窒素は生成されます。二酸化窒素の発生源は、工業用および家庭用ボイラーや自動車エンジンなどの燃焼過程で排出される一酸化窒素です。
特に、高圧で燃料を燃焼させる自動車エンジンが原因で、なかでもディーゼルエンジンは高濃度の排出源です。また、呼吸器など人の健康にも影響があり、二酸化窒素は代表的な大気汚染物質として知られています。
-
用途
合成中間体、酸化剤、硝酸の原料
-
構造
二酸化窒素の化学式は、NO2で表されます。C2v対称性を有する曲がった分子です。窒素原子と酸素原子の結合長は119.7pmであり、1と2の間の結合次数とも一致しています。二酸化窒素の結合角と結合長は、対応するカチオン (NO2+) とアニオン (NO2-) の中間の値を取っています。
-
合成法
工業的に二酸化窒素は、の触媒酸化で生じる一酸化窒素に、空気 (酸素) を混ぜて反応させることにより製造されます。銀や銅を濃硝酸と反応させても、二酸化窒素を生成可能です。
ただし、二酸化窒素は、さまざまな物質の燃焼や製造の過程で、意図せず副生成物として発生しています。例えば、燃焼により生じた一酸化窒素は、大気中で光反応を起こし酸化されて、二酸化窒素が生成します。
生物活動が原因で自然発生する場合もあり、地球規模では生物活動が発生源の大部分です。都市では移動発生源や固定発生源を含めて、二酸化窒素が高密度で生じており、大気汚染の要因の1つになっています。
-
説明
nitrogen dioxide is a reddish-brown
gas (or yellow liquid) with a strong, acrid odor. Nitrogen dioxide readily dimerizes to produce
N2O4.nitrogen dioxide are nonfl ammable, toxic gases.The federal government has established air quality standards for nitrogen dioxide
at 0.053 partsper million (ppm), which equals 100μg (micrograms) per cubic meter.Nitrogen dioxide is highly soluble in water and forms nitric acid (HNO3), and nitric oxide
is slightly soluble and forms nitrous acid (HNO2).
Nitrogen dioxide is
a strong oxidizing agent and causes corrosion.Nitrogen dioxide is used as an oxidizing agent, a catalyst in oxidation reactions, an inhibitor,
as a nitrating agent for organic reactions, as a flour bleaching agent, and in increasing the
wet strength of paper.
-
化学的特性
Nitrogen dioxide (and nitrogen tetroxide, the
solid dimer) is a dark brown gas (above 21 C) or a yellow,
fuming liquid or colorless solid with a pungent, acrid odor.
The solid form is colorless below about 11 C; it is found
structurally as N2O4.
-
物理的性質
Reddish-brown gas; pungent irritating odor; liquefies to a yellow liquid at 21.2°C; liquefies under pressure to a brown fuming liquid, commercially known as nitrogen tetroxide which actually is an equilibrium mixture of nitrogen dioxide and dinitrogen tetroxide, N2O4; converts to a colorless crystalline solid at -11.2°C; refractive index 1.40 at 20°C; density of gas in air 1.58 (air=1); density of liquid 1.449 g/mL at 20°C; critical temperature 158.2°C; critical pressure 99.96 atm; decomposes in water forming nitric acid; reacts with alkalies; soluble in concentrated nitric and sulfuric acids; soluble in chloroform and carbon disulfide.
-
天然物の起源
Nitrogen dioxide is an intermediate in producing nitric acid. It also is used in the lead chamber process for making sulfuric acid. It is used as a nitrating and oxidizing agent, in rocket fuels, in the manufacture of hemostatic cotton and other oxidized cellulose compounds, and in bleaching flour. Nitrogen dioxide occurs in trace concentrations in the atmosphere due to oxidation of nitric oxide in air. It also is found in exhaust gases of internal combustion engines, in industrial waste gases from plants using nitric acid, and in cigarette smoke. Brown color of smog in many industrial urban areas is attributed to nitrogen dioxide.
-
来歴
nitrogen dioxide was prepared in 1772 by Joseph Priestley
(1733–1804) and described in his volumes Experiments and Observations of Different Kinds
of Air published between 1774 and 1786. Priestley called nitric oxide nitrous air, nitrogen
dioxide nitrous acid vapor, and nitrous oxide phlogisticated nitrous air, but also referred to the
dioxide. Priestley prepared nitric oxide by reacting nitric acid with a metal such as copper:
3Cu(s) + 8HNO3(aq) → 2NO(g) + 3Cu(NO3)2(aq) + 4H2O(l).
-
使用
Nitrogen dioxide is an intermediate in producing nitric acid. It also is used in the lead chamber process for making sulfuric acid. It is used as a nitrating and oxidizing agent, in rocket fuels, in the manufacture of hemostatic cotton and other oxidized cellulose compounds, and in bleaching flour. Nitrogen dioxide occurs in trace concentrations in the atmosphere due to oxidation of nitric oxide in air. It also is found in exhaust gases of internal combustion engines, in industrial waste gases from plants using nitric acid, and in cigarette smoke. Brown color of smog in many industrial urban areas is attributed to nitrogen dioxide.
-
定義
A brown gas produced by the dissociation
of dinitrogen tetroxide (with which it is in
equilibrium), the dissociation being complete
at 140°C. Further heating causes dissociation
to colorless nitrogen monoxide
and oxygen:
2NO2(g) = 2NO(g) + O2(g)
Nitrogen dioxide can also be made by
the action of heat on metal nitrates (not the
nitrates of the alkali metals or some of the
alkaline-earth metals).
-
一般的な説明
A reddish brown gas or yellowish-brown liquid when cooled or compressed. Shipped as a liquefied gas under own vapor pressure. Vapors are heavier than air. Toxic by inhalation (vapor) and skin absorption. Noncombustible, but accelerates the burning of combustible materials. Cylinders and ton containers may not be equipped with a safety relief device.
-
空気と水の反応
Combines with oxygen to form NITROGEN DIOXIDE, a brown gas that is deadly poisonous [Merck 11th ed. (1989]. Decomposes in water to form nitric acid and nitric oxide, reacts with alkalis to form nitrate and nitrites [Merck 11th ed. 1989]. The liquid nitrogen oxide is very sensitive to detonation, in the presence of water.
-
反応プロフィール
NITROGEN DIOXIDE (nitrogen peroxide) is a strong oxidizing agent. Powdered aluminum burns in the vapor of carbon disulfide, sulfur dioxide, sulfur dichloride, nitrous oxide, nitric oxide, or nitrogen peroxide [Mellor 5:209-212. 1946-47]. Boron trichloride reacts energetically with nitrogen peroxide, phosphine, or fat and grease [Mellor 5:132. 1946-47]. Nitrogen peroxide and acetic anhydride reacted to form tetranitromethane, but resulted in an explosion [Van Dolah 1967]. Nitrogen peroxide forms explosive mixtures with incompletely halogenated hydrocarbons [Chem. Eng. News 42(47):53. 1964]. During an experiment to produce lactic acid by oxidizing propylene with nitrogen peroxide, a violent explosion occurred. These mixtures (olefins and nitrogen peroxide) form extremely unstable nitrosates or nitrosites [Comp. Rend. 116:756. 1893]. Contact of very cold liquefied gas with water may result in vigorous or violent boiling of the product and extremely rapid vaporization due to the large temperature differences involved. If the water is hot, there is the possibility that a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if liquid gas contacts water in a closed container [Handling Chemicals Safely 1980]. Corrosive to steel when wet, but may be stored in steel cylinders when moisture content is 0.1% or less.
-
危険性
Inhalation may be fatal. Can react strongly
with reducing materials. Lower respiratory tract
irritant. Questionable carcinogen.
-
健康ハザード
The acute toxicity of nitrogen dioxide by inhalation is high. Inhalation may cause shortness of
breath and pulmonary edema progressing to respiratory illness, reduction in the blood's oxygen
carrying capacity, chronic lung disorders and death; symptoms may be delayed for hours and
may recur after several weeks. Toxic effects may occur after exposure to concentrations of 10
ppm for 10 min and include coughing, chest pain, frothy sputum, and difficulty in breathing.
Brief exposure to 200 ppm can cause severe lung damage and delayed pulmonary edema, which
may be fatal. Nitrogen dioxide at concentrations of 10 to 20 ppm is mildly irritating to the eyes;
higher concentrations of the gas and liquid NO2-N2O4 are highly corrosive to the skin, eyes, and
mucous membranes. Nitrogen dioxide can be detected below the permissible exposure limit by
its odor and irritant effects and is regarded as a substance with adequate warning properties.
Animal testing indicates that nitrogen dioxide does not have carcinogenic or reproductive effects.
It does produce genetic damage in bacterial and mammalian cell cultures; however, most studies
in animals indicate that it does not produce heritable genetic damage.
-
燃焼性と爆発性
Nitrogen dioxide is not combustible (NFPA rating = 0) but is a strong oxidizing
agent and will support combustion. Cylinders of NO2 gas exposed to fire or intense
heat may vent rapidly or explode.
-
化学性质
水と反応して硝酸を生成
-
使用用途
二酸化窒素の使用用途として、分析化学の試料溶解剤や分解剤が挙げられます。また、等の窒素化合物の原料および合成中間体や、漂白剤、触媒、有機化合物のニトロ化剤にも用いられます。さらに、酸化剤としての爆薬の原料や重合禁止剤にも使用可能です。
発煙硝酸などのロケット燃料の酸化剤としても、二酸化窒素は利用されます。実際に、ロケットのタイタン、ジェミニ計画の打ち上げ、スペースシャトルのサイドスラスター、惑星に送った無人宇宙探査機などで使用されました。
-
二酸化窒素による環境汚染
二酸化窒素は、大気汚染防止法で特定物質に指定されています。1970年代頃までは、自動車保有台数の増加に伴って、二酸化窒素による汚染が進んでいました。その後、排出ガス規制の効果もあり、年平均値は長期的に横ばいの状況が続いています。幹線道路の沿線を中心に、環境基準が達成できていない状況です。
ヒトに対して、主に呼吸器系統の健康影響が報告されています。1日の二酸化窒素の平均値が、0.04〜0.06ppmの範囲内かそれ以下であるべきと、環境基準は定められています。
-
材料の用途
When dry (0.1 percent moisture or less), nitro�gen dioxide is not corrosive to mild steel at or�dinary temperatures and pressures. Numerous
metals and alloys such as carbon steel, stainless
steel, aluminum, nickel, and Inconel are satis�factory for handling and storage. Under wet
conditions, stainless steels resistant to about 60
percent nitric acid serve best.
Equipment parts, such as valve stems, which
are partly in contact with the atmosphere,
should be stainless steel with sufficient chro�mium content to resist corrosion caused by
leaks through stuffing boxes. Good quality ce�ramic bodies and Pyrex are satisfactory for han�dling wet or dry nitrogen dioxide.
Among the plastics, Teflon and Kel-F films
are most satisfactory. Koroseal and Saran are
useful but have a limited service life. In general,
the vinyl plastics do not hold up well with nitro�gen dioxide. Asbestos and asbestos-graphite are
satisfactory for valve stuffing boxes. Koroseal
has given reasonably good service in this use.
For use on pipe threads, graphite-disodium sili�cate (waterglass) is recommended, and hydro�carbon lubricants should be avoided.
-
安全性プロファイル
Experimental poison by
inhalation. Moderately toxic to humans by
inhalation. An experimental teratogen.
Other experimental reproductive effects.
Human systemic effects by inhalation:
pulmonary vascular resistance changes,
cough, dpspnea, and other pulmonary
changes. Mutation data reported. Violent
reaction with cyclohexane, F2,
formaldehyde, alcohols, nitrobenzene,
petroleum, toluene. When heated to
decomposition it emits toxic fumes of NOx.
See also NITRIC OXIDE.
-
職業ばく露
Nitrogen dioxide is found in automotive
and diesel emissions. Nitrogen dioxide is an industrial
chemical used as an intermediate in nitric and sulfuric acid
manufacture; it is used in the nitration of organic compounds;
it is used as an oxidizer in liquid propellant rocket
fuel combinations. It is also used in firefighting, welding
and brazing.
-
貯蔵
Cylinders of nitrogen dioxide should be stored and used
in a continuously ventilated gas cabinet or fume hood.
-
合成方法
一酸化窒素の酸化
-
輸送方法
UN1067/124 Dinitrogen tetroxide, Hazard Class:
2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive
material, Inhalation Hazard Zone A. UN1975 Nitric oxide
and dinitrogen tetroxide mixtures or Nitric oxide and
nitrogen dioxide mixtures, Hazard Class: 2.3; Labels:
2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material,
Inhalation Hazard Zone A. Cylinders must be transported
in a secure upright position, in a well-ventilated truck.
Protect cylinder and labels from physical damage. The
owner of the compressed gas cylinder is the only entity
allowed by federal law (49CFR) to transport and refill
them. It is a violation of transportation regulations to refill
compressed gas cylinders without the express written permission
of the owner.
-
不和合性
A strong oxidizer. Reacts violently with
combustible matter, chlorinated hydrocarbons; ammonia,
carbon disulfide; reducing materials. Reacts with water,
forming nitric acid and nitric oxide. Attacks steel in the
presence of moisture.
-
廃棄物の処理
Destroy by incineration with
the addition of hydrocarbon fuel, controlled in such a way
that combustion products are elemental nitrogen, CO2, and
water. Consult with environmental regulatory agencies for
guidance on acceptable disposal practices. Generators of
waste containing this contaminant (≥100 kg/mo) must conform
with EPA regulations governing storage, transportation,
treatment, and waste disposal.




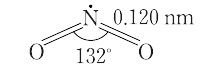 "
"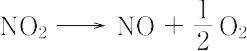 "のように分解する.硝酸の製造,硝化剤,酸化剤,アクリレートの重合禁止剤として用いられる.毒性が強く,吸入すると肺がおかされ,200 ppm 程度でも死に至るおそれがある.[CAS 10102-44-0][別用語参照]大気汚染,窒素酸化物
"のように分解する.硝酸の製造,硝化剤,酸化剤,アクリレートの重合禁止剤として用いられる.毒性が強く,吸入すると肺がおかされ,200 ppm 程度でも死に至るおそれがある.[CAS 10102-44-0][別用語参照]大気汚染,窒素酸化物