-
外観
無色澄明の液体
-
性質
トリクロロエチレンの沸点は86.7°C、融点は-86.4°Cで、発火点は420°Cです。常温では不燃性や揮発性を示し、クロロホルムのような甘い香りを持っています。
エタノール、ジエチルエーテル、クロロホルムのような、多くの有機溶媒によく溶けます。金属の存在下では、長時間は安定していません。高温ではさらに不安定になります。そのため、市販されているトリクロロエチレンには、添加剤が用いられています。
-
溶解性
水に難溶 (0.1g/100ml水, 20℃), アルコール, エーテル等各種有機溶剤と混和。エタノール及びジエチルエーテルに極めて溶けやすく、水に溶けにくい。
-
解説
トリクロロエテン.トリクレンともいう.1,1,2,2-テトラクロロエタンを水酸化カルシウムまたはアルカリで脱塩化水素すると得られる.クロロホルム臭のある無色の液体.融点-86 ℃,沸点88 ℃.d15 1.4397.n20D 1.4782.水に不溶,多くの有機溶媒に可溶.不燃性で有毒.日光にはあまり安定ではない.ゴム,油脂,樹脂,塗料の溶剤であり,消火剤成分や十二指腸虫駆除剤にも用いられる.中枢神経の抑制作用があり,麻ひを起こすことがある.LD50 2402 mg/kg(マウス,経口).ヒトに対する最小致死量は857 mg/kg.
-
用途
金属部のクリーニング、脱脂、接着剤中の溶剤
-
用途
溶剤、抽出溶媒。
-
用途
溶剤、抽出溶媒、洗浄剤。
-
用途
代替フロンガス合成原料、機械部品?電子部品等脱脂洗浄剤、羊毛?皮革洗浄剤、油脂?樹脂?ゴム工業用溶剤、染料?塗料溶剤、試薬、金属洗浄剤、溶剤(生ゴム,塗料,油脂,ピッチ)、フロンガス製造原料
-
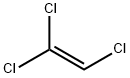
構造
トリクロロエチレンは、エチレンの3個の水素原子が塩素原子になった有機塩素化合物です。有機塩素化合物とは、炭素を含む化合物にが結合した物質のことです。
化学式はC2HCl3と表されます。20°Cでの密度は1.46g/cm3です。
-
合成法
1. アセチレンを用いたトリクロロエチレンの合成法
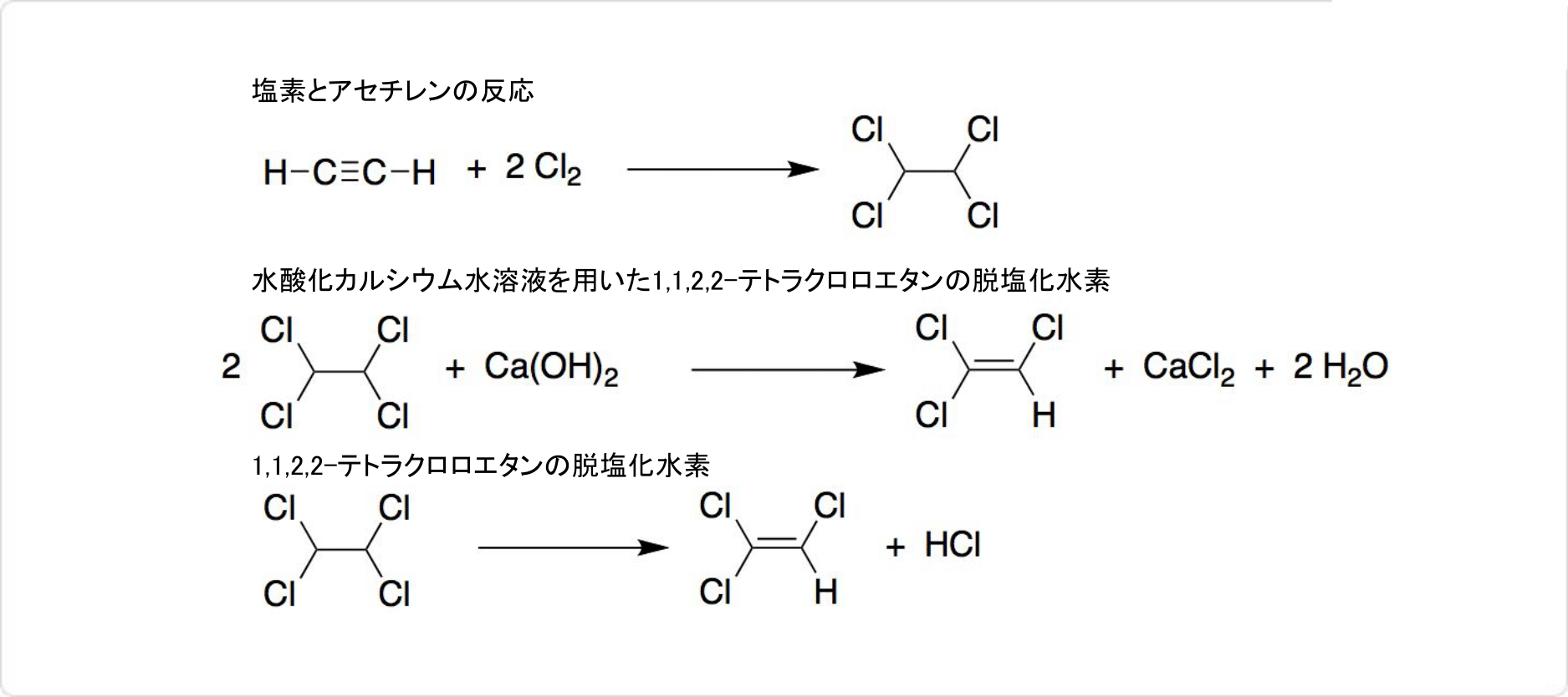
トリクロロエチレンのアセチレンからの合成
1970年代初頭以前にトリクロロエチレンは、アセチレンから2段階の反応で製造されていました。90℃で塩化鉄 (III) 触媒を用いて、塩素とアセチレンの反応によって、1,1,2,2-テトラクロロエタンが得られます。
水酸化カルシウム水溶液を用いた1,1,2,2-テトラクロロエタンの脱塩化水素反応によって、トリクロロエチレンが生成可能です。1,1,2,2-テトラクロロエタンの脱塩化水素は、塩化カルシウムや塩化バリウムなどの触媒を使用して、気相中で300〜500℃に熱しても合成できます。
2. エチレンを用いたトリクロロエチレンの合成法
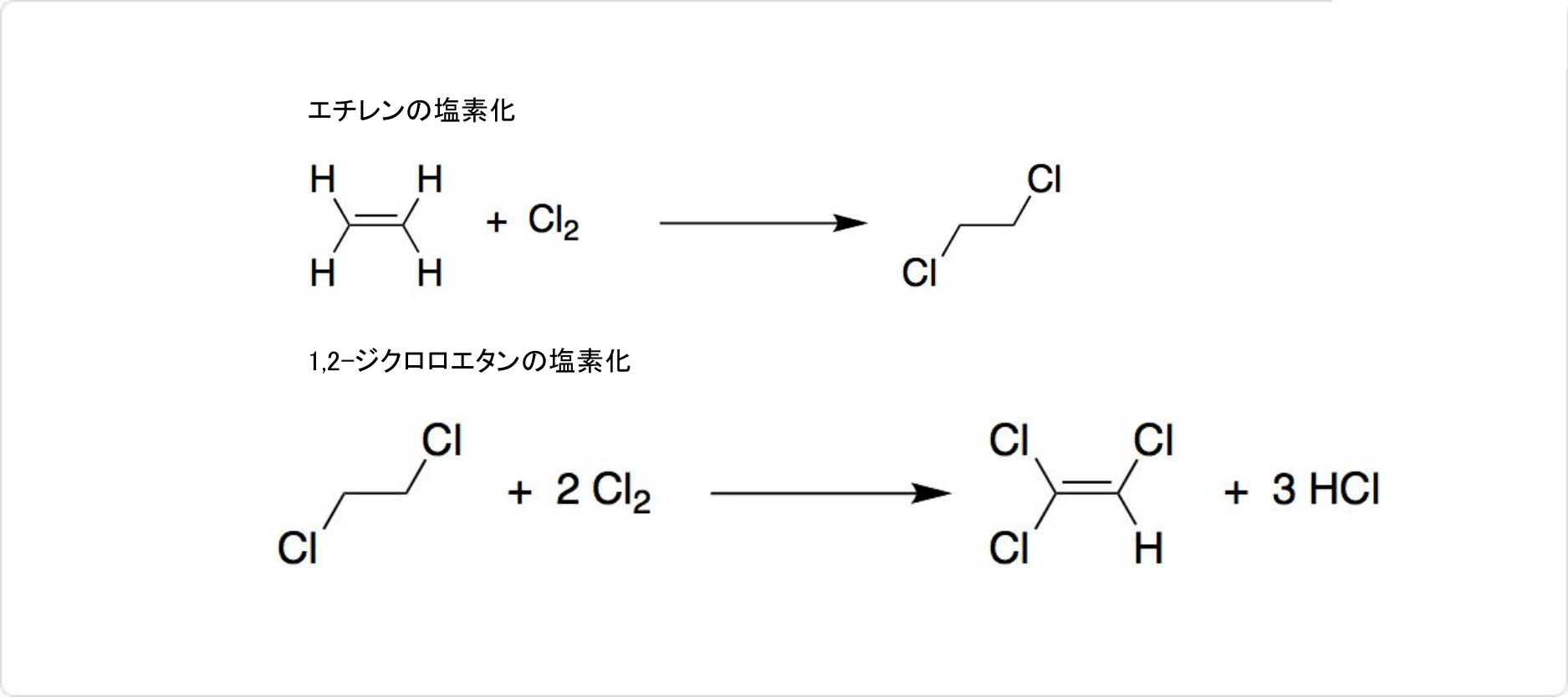
トリクロロエチレンのエチレンからの合成
現在は、エチレンから大部分のトリクロロエチレンを合成しています。触媒として塩化鉄 (III) を使ってエチレンを塩素化すると、1,2-ジクロロエタンを生成可能です。
1,2-ジクロロエタンに塩素を加えて、400℃付近で熱すると、トリクロロエチレンが生成します。1,2-ジクロロエタンの塩素化の触媒には、塩化アルミニウムと塩化カリウムの混合物のほか、多孔質の炭素も利用可能です。使用する塩素の量によっては、テトラクロロエチレンが副生しますが、蒸留で分離できます。
-
危険性
蒸気吸入や直接接触などでトリクロロエチレンが体内に入った場合、頭痛、めまい、おう吐などの中毒症状を引き起こします。また、肝臓や腎臓に障害を起こす可能性もあります。
トリクロロエチレンは、人体に対する毒性が強いです。環境に放出された場合には水に溶けにくく、土壌汚染や地下水汚染などの問題に繋がります。そのため、取り扱いには厳格な規制があります。
-
説明
Trichloroethylene (IUPAC), CHClCCl2, is a stable, low-boiling, colorless liquid with a chloroform-like odor. It is not corrosive to the common metals even in the presence of moisture. It is slightly soluble in water and is nonflammable. It is toxic by inhalation, with a TLV of 50 ppm and an IDLH of 1000 ppm in air. The FDA has prohibited its use in foods, drugs, and cosmetics. The four-digit UN identification number is 1710. The NFPA 704 designation is health 2, flammability 1, and reactivity 0. Its primary uses are in metal degreasing, dry cleaning, as a refrigerant and fumigant, and for drying electronic parts.
-
化学的特性
Trichloroethylene, a colorless (often dyed
blue), nonflammable, noncorrosive liquid that has the
“sweet” odor characteristic of some chlorinated hydrocarbons.
The Odor Threshold is 25-50 ppm.
-
物理的性質
Clear, colorless, watery-liquid with a chloroform-like odor. Odor threshold concentrations
determined in air were 21.4 ppmv (Leonardos et al., 1969) and 3.9 ppmv (Nagata and Takeuchi,
1990). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40
°C were 10 and 2.6 mg/L, respectively (Alexander et al., 1982).
-
使用
Trichloroethylene is used as a solvent, in drycleaning, in degreasing, and in limited use asa surgical anesthetic.
-
調製方法
TCE has been in commercial use for almost 60 years. TCE
has been used as a solvent because of its powerful ability to dissolve fats, greases, and waxes. It has been widely used in
the dry cleaning industry and as a metal degreaser and in the
electronic components industry where workers have been
observed using it as a cleaning solvent without any protective
equipment, thus allowing uncontrolled skin contact and
inhalation exposures.
-
定義
ChEBI: A member of the class of chloroethenes that is ethene substituted by chloro groups at positions 1, 1 and 2.
-
一般的な説明
A clear colorless volatile liquid having a chloroform-like odor. Denser than water and is slightly soluble in water. Noncombustible. Used as a solvent, fumigant, in the manufacture of other chemicals, and for many other uses.
-
空気と水の反応
Slightly soluble in water.
-
反応プロフィール
Trichloroethylene has been determined experimentally that mixtures of finely divided barium metal and a number of halogenated hydrocarbons possess an explosive capability. Specifically, impact sensitivity tests have shown that granular barium in contact with monofluorotrichloromethane, trichlorotrifluoroethane, carbon tetrachloride, Trichloroethylene, or tetrachloroethylene can detonate (ASESB Pot. Incid. 39. 1968; Chem. Eng. News 46(9):38. 1968). Trichloroethylene has been determined experimentally that a mixture of beryllium powder with carbon tetrachloride or with Trichloroethylene will flash or spark on heavy impact (ASESB Pot. Incid. 39. 1968). A mixture of powdered magnesium with Trichloroethylene or with carbon tetrachloride will flash or spark under heavy impact (ASESB Pot. Incid, 39. 1968).
-
健康ハザード
The toxic effects manifested in humansfrom inhaling trichloroethylene vapors areheadache, dizziness, drowsiness, fatigue, andvisual disturbances. A 2-hour exposure to a1000-ppm concentration affected the visualperception. Higher concentrations can pro duce narcotic effects. Heavy exposures maycause death due to respiratory failure or car diac arrest. A 4-hour exposure to 8000 ppmwas lethal to rats. Chronic exposure causedincrease in kidney and liver weights in testanimals.
The symptoms of poisoning from oralintake of trichloroethylene are nausea, vom iting, diarrhea, and gastric disturbances. Theacute oral toxicity, however, is low. Theoral LD50 value in mice is in the range2500 mg/kg. Trichloroethylene metabolizesto trichloroacetic acid, which is excreted inthe urine.
Although trichloroethylene exhibits lowtoxicity, its metabolite trichloroethanol, andoxidative degradation products phosgene,COCl2, and chlorine, can cause severe unex pected health hazards. Kawakami andassociates (1988) reported a case of Steven–Johnson syndrome in a worker in a printingfactory. In another case, fire on a stove in ametal-degreasing workplace produced phos gene and chlorine inhalation, which causeddyspnea, fever, and fatigue.
Trichloroethylene exhibited evidence ofcarcinogenicity in laboratory animals. Oraladministration produced liver tumors, whileinhalation caused lung and blood tumors inmice and rats.
-
火災危険
Special Hazards of Combustion Products: Toxic and irritating gases are produced in fire situations.
-
使用用途
トリクロロエチレンは、油脂類やなどのなどを溶解するため、金属部品や電子部品の脱脂洗浄が主な用途です。さらに、ゴムや樹脂を溶解するため、中の溶剤としても使用され、染料や塗料の製造時に工業用溶剤として用いられています。
そのほか、代替フロン合成原料として利用可能です。つまり、フロンガスの製造にも使用されています。
また、水洗いできない衣類や羊毛、革製品の油分を洗浄するドライクリーニングに使用可能です。それ以外にも、水質基準などの測定時に比較対象の濃度標準液として使われますが、使用量は多くありません。
-
化学反応性
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
-
接触アレルゲン
Trichloroethylene is a chlorinated hydrocarbon used as
a detergent or solvent for metals, oils, resins, sulfur, and
as general degreasing agent. It can cause irritant contact
dermatitis, generalized exanthema, Stevens-Johnson-
like syndrome, pustular or bullous eruption, scleroderma,
as well as neurological and hepatic disorders.
-
職業ばく露
Trichloroethylene is used as a vapor
degreaser of metal parts, as a solvent; and as a drug; It is also
used for extracting caffeine from coffee, as a dry-cleaning
agent; and as a chemical intermediate in the production of
pesticides; in making waxes, gums, resins, tars, paints,
varnishes, and specific chemicals; such as chloroacetic acid.
-
発がん性
Trichloroethylene is reasonably anticipated to be a human carcinogen based on limited evidence of carcinogenicity from studies in humans, sufficient evidence of carcinogenicity from studies in experimental animals, and information from studies on mechanisms of carcinogenesis.
-
代謝経路
From the photooxidation reaction medium (1) of
trichloroethylene, the formation of dichloroacetyl
chloride, CO, phosgene, and pentachloroethane and
their conversion to the final product, CO2, are
identified. By the second TiO2 photocatalyst reaction
(2), trichloroacetaldehyde, dichloroacetyl chloride, CO,
and phosgene with the new identified intermediates
oxalyl chloride, trichloroacetyl chloride, and
trichloroacetic acid are observed.
-
輸送方法
UN1710 Trichloroethylene, Hazard Class: 6.1;
Labels: 6.1-Poisonous materials.
-
純化方法
Tricloroethylene undergoes decomposition in a similar way as CHCl3, giving HCl, CO, COCl2 and organic products. It reacts with KOH, NaOH and 90% H2SO4, and forms azeotropes with water, MeOH, EtOH, and acetic acid. It is purified by washing successively with 2M HCl, water and 2M K2CO3, then dried with K2CO3 and CaCl2, then fractionally distilled before use. It has also been steam distilled from 10% Ca(OH)2 slurry, most of the water being removed from the distillate by cooling to -30o to -50o and filtering off the ice through chamois skin: the trichloroethylene is then fractionally distilled at 250mm pressure and collected in a blackened container. [Carlisle & Levine Ind Eng Chem (Anal Ed) 24 1164 1932, Beilstein 1 IV 712.]
-
不和合性
Contact with strong caustics causes
decomposition and the production of highly toxic and flammable
dichloroacetylene. Violent reaction with chemically
active metals; powders, or shavings, such as aluminum,
barium, lithium, sodium, magnesium, and titanium. Violent
reaction with aluminum in the presence of dilute hydrochloric
acid. Thermal decomposition of trichloroethylene,
due to contact with hot metal or UV radiation, forms hazardous
products including chlorine gas, hydrogen chloride;
and phosgene. Keep this chemical away from high temperatures,
such as arc welding or cutting, unshielded resistance
heating; open flames; and high intensity UV light. Slowly
decomposed by light in presence of moisture, with formulation
of hydrochloric acid.
-
廃棄物の処理
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal.
Incineration, preferably after mixing with another combustible
fuel. Care must be exercised to assure complete combustion
to prevent the formation of phosgene. An acid
scrubber is necessary to remove the halo acids produced.
An alternative to disposal for TCE is recovery and
recycling.