-
外観
無色澄明の液体
-
性質
トリエチルアミンは水、エタノール、およびほとんどの有機溶媒に非常によく溶けます。沸点は89℃、融点は-114.7℃、20℃における密度は0.726g/mLです。トリエチルアミンは強い刺激臭があり、しばしばアンモニアや魚の臭いに似ていると表現されます。
その化学的特性は、主に窒素原子に2個の水素原子が結合したアミン官能基の存在に起因しています。窒素原子上の電子が1対であるため、トリエチルアミンは強い塩基性を示します。
また、トリエチルアミンは強い求核剤であることが知られており、電子対を供与して求電子剤と新たな化学結合を形成します。このため、トリエチルアミンは有機合成の試薬として幅広く使用されています。
トリエチルアミンの毒性は高くはありませんが、大量に摂取したり吸い込んだりすると有害な場合があります。また、トリエチルアミンは可燃性であるため、取り扱いに注意が必要です。
-
溶解性
水 18.7℃未満では混和,18.7℃以上では僅かにとける。 アルコール, アセトンに易溶。エタノール及びアセトンに極めて溶けやすく、水に溶けにくい。
-
解説
トリエチルアミン,水に溶解しにくい塩基性液体.融点-114.8 ℃,沸点89.5 ℃.d420"0.7275.nD20"1.4010.pKa 10.72(25 ℃).塩基性触媒,脱ハロゲン化水素剤として縮合反応にしばしば使われる.皮膚,粘膜をはげしく刺激する.
森北出版「化学辞典(第2版)
-
用途
有機溶剤、ゴム製造の促進剤
-
用途
医薬?染料?農薬?塗料原料 (NITE CHRIP)
-
構造
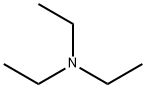
トリエチルアミンは3級アミンであり、窒素原子(-N)に結合した3つのエチル基(-C2H5)を持っています。
窒素原子は電子の単独対を持っており、これがトリエチルアミンの性質特徴づけています。窒素原子がプロトン(H+)を受け入れて正電荷のアンモニウムイオン(C2H5)3NH+を形成することができるため、トリエチルアミンは強塩基性を示します。
-
製造
トリエチルアミン,第三級アミンの一つ.塩化エチルにアルコール性アンモニアを作用させてつくる.
-
主な用途/役割
エポキシ樹脂の硬化剤として使用される。
-
毒性
LD50 460 mg/kg(ラット,経口).
-
化学的特性
Triethylamine is a colorless to yellowish liquid with a strong ammonia to fish-like odor. It is a base commonly used in organic chemistry to prepare esters and amides from acyl chlorides. Like other tertiary amines,it catalyzes the formation of urethane foams and epoxy resins.
-
物理的性質
Clear, colorless to light yellow flammable liquid with a strong, penetrating, ammonia-like odor.
Experimentally determined detection and recognition odor threshold concentrations were <400
μg/m3 (<100 ppbv) and 1.1 mg/m3 (270 ppbv), respectively (Hellman and Small, 1974). An odor
threshold concentration of 0.032 ppbv was determined by a triangular odor bag method (Nagata
and Takeuchi, 1990).
-
使用
Triethylamine is a base used to prepare esters and amides from acyl chlorides as well as in the synthesis of quaternary ammonium compounds. It acts as a catalyst in the formation of urethane foams and epoxy resins, dehydrohalogeantion reactions, acid neutralizers for condensation reactions and Swern oxidations. It finds application in reverse phase high-performance liquid chromatography (HPLC) as a mobile-phase modifier. It is also used as an accelerator activator for rubber, as a propellant, as a corrosion inhibitor, as a curing and hardening agent for polymers and for the desalination of seawater. Furthermore, it is used in the automotive casting industry and the textile industry.
-
調製方法
Triethylamine is prepared by a vapor phase reaction of ammonia with ethanol or reaction of N,N-diethylacetamide with lithium aluminum hydride (Windholz et al 1983). It may also be produced from ethyl chloride and ammonia under heat and pressure (Hawley 1981) or by vapor phase alkylation of ammonia with ethanol (HSDB 1988). U.S. production is estimated at greater than 22,000 tons in 1972 (HSDB 1988).
-
定義
ChEBI: Triethylamine is a tertiary amine that is ammonia in which each hydrogen atom is substituted by an ethyl group.
-
主な応用
Triethylamine (TEA, Et3N) is an aliphatic amine. It is used to catalytic solvent in chemical synthesis; accelerator activators for rubber; wetting, penetrating, and waterproofing agents of quaternary ammonium types; curing and hardening of polymers (e.g., corebinding resins); corrosion inhibitor; propellant.
Triethylamine has been used during the synthesis of:
5′-dimethoxytrityl-5-(fur-2-yl)-2′-deoxyuridine
3′-(2-cyanoethyl)diisopropylphosphoramidite-5′-dimethoxytrityl-5-(fur-2-yl)-2′-deoxyuridine
polyethylenimine600-β-cyclodextrin (PEI600-β-CyD)
It may be used as a homogeneous catalyst for the preparation of glycerol dicarbonate, via transesterification reaction between glycerol and dimethyl carbonate (DMC).
-
安全性
トリエチルアミンは皮膚、眼に対する腐食性を示すほか、特定標的臓器毒性(単回ばく露)の中枢神経系で区分1に分類されています。また前述の通り、や腐った魚のような強い不快臭を発する物質です。したがってトリエチルアミンを使用する際は保護具を着用するのはもちろんのこと、漏洩対策も確実に行う必要があります。
トリエチルアミンを使用する前は作業の危険性を評価するとともに、廃棄手順も明確に定めておくことが推奨されます。
-
一般的な説明
Triethylamine appears as a clear colorless liquid with a strong ammonia to fish-like odor. Flash point 20°F. Vapors irritate the eyes and mucous membranes. Less dense (6.1 lb / gal) than water. Vapors heavier than air. Produces toxic oxides of nitrogen when burned.
-
反応プロフィール
Triethylamine reacts violently with oxidizing agents. Reacts with Al and Zn. Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
-
健康ハザード
Vapors irritate nose, throat, and lungs, causing coughing, choking, and difficult breathing. Contact with eyes causes severe burns. Clothing wet with chemical causes skin burns. Triethylamine may also be irritating to skin and mucous membranes (Windholz et al 1983).
-
火災危険
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
-
使用用途
トリエチルアミンは第三級アミンの一種で、アセトンや、などの幅広い有機溶媒に可溶な塩基であるため、合成反応で広く用いられています。
工業的には医薬品や染料の中間体、ポリマー合成、農薬などに用いられているほか、とイソシアネート樹脂のガス硬化反応(コールドボックス法)における触媒として使用されることもあります。
食品業界においてトリエチルアミンはスルメイカや魚類の中にも存在していたり、欧米では肉製品や冷凍乳製品類などの風味向上などの目的で添加されたりしています。
-
化学性质
常温では水に微溶
-
工業用途
Triethylamine is used as an anti-livering agent for urea- and melamine-based enamels and in the recovery of gelled paint vehicles (HSDB 1988). It is also used as a catalyst for polyurethane foams, a flux for copper soldering, and as a catalytic solvent in chemical synthesis (Hawley 1981). Triethylamine is used in accelerating activators for rubber; as a corrosion inhibitor for polymers; a propellant; wetting, penetrating, and waterproofing agent of quaternary ammonium compounds; in curing and hardening of polymers (i.e. core-binding resins); and as a catalyst for epoxy resins (Hamilton and Hardy, 1974).
-
安全性プロファイル
Moderately toxic by
ingestion and skin contact. Mildly toxic by
inhalation. Human systemic effects: visual
field changes. Experimental reproductive
effects. Mutation data reported. A skin and
severe eye irritant. Can cause kidney and
liver damage. A very dangerous fire hazard
when exposed to heat, flame, or oxidizers.
Explosive in the form of vapor when
exposed to heat or flame. Complex with
dinitrogen tetraoxide explodes below 0°C
when undduted with solvent. Exothermic
reaction with maleic anhydride above 150°C.
Can react with oxidzing materials.
Incompatible with N2O4. To fight fire, use
CO2, dry chemical, alcohol foam. When
heated to decomposition it emits toxic
fumes of NOx.
-
職業ばく露
Triethylamine is and aliphatic amine
used as a solvent; corrosion inhibitor; in chemical synthesis;
and accelerator activators; paint remover; base in methylene
chloride or other chlorinated solvents. TEA is used to solubilize
2,4,5-T in water and serves as a selective extractant in
the purification of antibiotics. It is used to manufacture quaternary
ammonia compounds and octadecyloxymethyltriethylammonium
chloride; an agent used in textile treatment.
-
概要
トリエチルアミンは窒素に3つのエチル基(C2H5)が結合した第三級アミンで、強いアンモニア臭のある無色透明の液体です。化学式は (C2H5)3N で表され、一般に「TEA」と略されます。
トリエチルアミンはやなどの汎用的な有機溶媒に溶解しやすい強塩基であり、工業用および実験用としてさまざまな用途に使用されています。
また、医薬品や染料中間体などの分野を中心に、工業的にも幅広く使われています。
一方で、トリエチルアミンは悪臭を持つほか、皮膚、眼に対する刺激性が高いうえ。そのため、取り扱い時は漏洩、人体への接触がないこと、火災や爆発を引き起こさぬよう安全対策が求められます。
-
発がん性
TEA was not mutagenic in bacterial assays,
but it did cause aneuploidy and chromosome
aberrations in rats.
-
製造方法
トリエチルアミンは、主にエチレン、アンモニア、エタノールを原料として生産されます。
このプロセスは以下の工程で進行します。
①中間体(エチレンジアミン)の合成
エチレンとアンモニアを、温度約200~250℃、圧力約1~5MPaの条件で混合します。この混合物をアルミナやシリカアルミナといった触媒上に通すと、エチレンジアミンが生成します。
H2C=CH2 + NH3 → H2NCH2CH2NH2
②トリエチルアミン合成
エチレンジアミンを、ルイス酸などの別の触媒の存在下でエタノールと反応させることでトリエチルアミンが生成します。
H2NCH2CH2NH2 + 2 C2H5OH → (C2H5)3N + H2O + C2H4
その後、蒸留または抽出によって、反応混合物からTEAを分離することが可能です。
-
環境運命予測
Photolytic. Low et al. (1991) reported that the photooxidation of aqueous tertiary amine
solutions by UV light in the presence of titanium dioxide resulted in the formation of ammonium
and nitrate ions.
Chemical/Physical. Triethylamine reacted with NOx in the dark to form diethylnitrosamine. In
an outdoor chamber, photooxidation by natural sunlight yielded the following products:
diethylnitramine, diethylformamide, diethylacetamide, ethylacetamide, diethylhydroxylamine,
ozone, acetaldehyde, and peroxyacetyl nitrate (Pitts et al., 1978).
-
代謝
There have been few studies on the metabolism of industrially important aliphatic amines such as triethylamine. It is generally assumed that amines not normally present in the body are metabolized by monoamine oxidase and diamine oxidase (histaminase).
Ultimately ammonia is formed and will be converted to urea. The hydrogen peroxide formed is acted upon by catalase and the aldehyde formed is thought to be converted to the corresponding carboxylic acid by the action of aldehyde oxidase (Beard and Noe 1981).
-
輸送方法
UN1296 Triethylamine, Hazard Class: 3; Labels:
3-Flammable liquid, 8-Corrosive material.
-
合成方法
詳細な合成方法が文書からは判りません
-
純化方法
Dry triethylamine with CaSO4, LiAlH4, Linde type 4A molecular sieves, CaH2, KOH, or K2CO3, then distil it, either alone or from BaO, sodium, P2O5 or CaH2. It has also been distilled from zinc dust, under nitrogen. To remove traces of primary and secondary amines, triethylamine has been refluxed with acetic anhydride, benzoic anhydride, phthalic anhydride, then distilled, refluxed with CaH2 (ammonia-free) or KOH (or dried with activated alumina), and again distilled. Another purification method involved refluxing for 2hours with p-toluenesulfonyl chloride, then distilling. Grovenstein and Williams [J Am Chem Soc 83 412 1961] treated triethylamine (500mL) with benzoyl chloride (30mL), filtered off the precipitate, and refluxed the liquid for 1hour with a further 30mL of benzoyl chloride. After cooling, the liquid was filtered, distilled, and allowed to stand for several hours with KOH pellets. It was then refluxed with, and distilled from, stirred molten potassium. Triethylamine has been converted to its hydrochloride (see brlow), crystallised from EtOH (to m 254o), then liberated with aqueous NaOH, dried with solid KOH and distilled from sodium under N2. [Beilstein 4 H 99, 4 I 348, 4 II 593, 4 III 194, 4 IV 322.]
-
不和合性
A strong base. Violent reaction with
strong acids; halogenated compounds; and strong oxidizers.
Attacks some forms of plastics, rubber and coatings.
Corrosive to aluminum, zinc, copper, and their alloys in the
presence of moisture. Reaction with nitrosating agents
(e.g., nitrites, nitrous gases, and nitrous acid) capable of
releasing carcinogenic nitrosamines.
-
廃棄物の処理
Controlled incineration
(incinerator equipped with a scrubber or thermal unit to
reduce nitrogen oxides emissions).