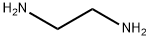-
外観
無色澄明の液体
-
性質
エチレンジアミンの化学式はNH2CH2CH2NH2で、エチレンの2つの炭素に結合する水素原子が1個ずつ、アミン基に置換された構造になっています。分子量は60.11で、密度は0.9g/cc、融点は8.5℃、沸点は117℃で、水、アルコールと自由に混和しますが、エーテルには微溶です。
強塩基性を示し、付着した生体組織部を腐食させます。また、加熱すると、窒素酸化物とアンモニアの有毒な煙が発生するため、取り扱いには注意が必要です。
不快なアンモニア臭があり、眼、鼻、のど、呼吸器系統に痛みと刺激を与え、まれに生命の危険を及ぼすこともあります。目や皮膚についた場合、多量の水で洗い流し、医師の手当てを受ける、濃厚な蒸気を吸入した場合は、新鮮な空気の場所に移動するこことが重要です。
-
溶解性
水, エタノールに易溶, エーテルに不溶。水及びエタノールに極めて溶けやすい。
-
解説
エチレンジアミン.工業的には,1,2-ジクロロエタンとアンモニアからつくられる.アンモニア臭をもつ無色の液体.融点10.8 ℃,沸点117 ℃.d2020 0.900.n20D 1.457.強塩基性で,亜硝酸によりエチレンオキシドになる.代表的なキレート試薬で,ほとんどの金属に二座配位して錯体を形成する.エチレンジアミン四酢酸(EDTA)として,また,殺菌剤,分析試薬,医薬品として用いられる.LD50 1.16 g/kg(ネズミ,経口).
-
用途
キレート剤?エポキシ樹脂硬化剤?殺菌剤?繊維加工剤 (防しわ剤,染料固着剤)?可塑剤?ゴム薬品合成原料;キレート化剤?繊維関係薬品(防しわ剤?界面活性剤?染料固着剤)?イオン交換樹脂?ゴム薬品原料;繊維関係,農薬原料,キレート化剤
-
効能
キレート剤, 安定化剤
-
主な用途/役割
ポリウレタン樹脂系接着剤の原料として使用される。
-
説明
Ethylenediamine is used in numerous industrial proces
ses as a solvent for casein or albumin, as a stabilizer
in rubber latex and as a textile lubricant. It can be found
in epoxy-resin hardeners, cooling oils, fungicides, and
waxes. Contact dermatitis from ethylenediamine is
almost exclusively due to topical medicaments. Occupational
contact dermatitis in epoxy-resin systems is
rather infrequent. Ethylenediamine can cross react with
triethylenetetramine and diethylenetriamine. Ethylenediamine
was responsible for sensitization in pharmacists
handling aminophylline suppositories, in nurses
preparing and administering injectable theophylline,
and in a laboratory technician in the manufacture of
aminophylline tab lets.
-
化学的特性
Ethylenediamine, a polyamine, is a strongly
alkaline, colorless, clear, thick liquid. Ammonia odor. A
solid below 8.5�℃. The Odor Threshold is 1.0 ppm
-
物理的性質
Clear, colorless, volatile, slight viscous, hygroscopic liquid with a sweet, ammonia-like odor. The
average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were
12 and 52 mg/L, respectively (Alexander et al., 1982).
-
使用
[Note—Edamine is the recommended contraction for the ethylenediamine radical.].
-
定義
ChEBI: An alkane-alpha,omega-diamine in which the alkane is ethane.
-
調製方法
The production of ethylene-1,2-diamine (EDA) is by the catalytic amination of
monoethanolamine or the reaction of aqueous ammonia with 1,2-dichloroethane
(Spitz 1979). U.S. Production is estimated at greater than 33,000 tons in 1975.
-
一般的な説明
Ethylenediamine is a linear aliphatic diamine, widely used as a building block in organic synthesis. It readily forms heterocyclic imidazolidine derivatives, because of its bifunctional nature, having two amines. Ethylenediamine is also utilized as a raw material for the synthesis of chelating agents, polymers, agrochemicals and pharmaceutical intermediates.
-
空気と水の反応
Highly flammable. Hygroscopic. Fumes in the air. Water soluble. Biodegrades readily.
-
反応プロフィール
A base. Highly reactive with many compounds. Can react violently with acetic acid, acetic anhydride, acrolein, acrylic acid, acrylonitrile, allyl chloride, carbon disulfide, chlorosulfonic acid, epichlorohydrin, ethylene chlorohydrin, hydrogen chloride, mesityl oxide, nitric acid, oleum, AgClO4, sulfuric acid, beta-propiolactone and vinyl acetate. Incompatible with strong acids, strong oxidizers (perchlorate salts), and chlorinated organic compounds. Ethylenediamine is also incompatible with halogenated organic compounds and metal halides. May react with nitromethane and diisopropyl peroxydicarbonate. May ignite on contact with cellulose nitrate. Readily absorbs carbon dioxide from the air to give crusty solid deposits. . Ethylenediamine reacts violently with ethylene chlorohydrin. (Lewis, R.J., Sr. 1992. Sax's Dangerous Properties of Industrial Materials, 8th Edition. New York: Van Nostrand Reinhold. pp. 1554.).
-
危険性
Toxic by inhalation and skin absorption,
strong irritant to skin and eyes. Flammable, moderate fire risk. Questionable carcinogen.
-
健康ハザード
Ethylenediamine is a severe skin irritant, producing sensitization, an allergic reaction andblistering on the skin. Pure liquid on contact with the eyes can damage vision. A25% aqueous solution can be injurious to theeyes. Inhalation of its vapors can producea strong irritation to the nose and respiratory tract leading to chemical pneumonitis and pulmonary edema. Such irritation inhumans with symptoms of cough and dis tressed breathing may be noted at concentrations of >400 ppm. Repeated exposure tohigh concentrations of this substance in airmay cause lung, liver, and kidney damage.The toxicity of this compound, however, is much less than that of ethylenimine.The acute oral toxicity value in animalswas low to moderate. An oral LD50 value inrats is 500 mg/kg (NIOSH 1986).
-
火災危険
Burning rate: 2.2 mm/minute. When exposed to heat or flame, the material has a moderate fire potential. The material can react readily with oxidizing materials. Containers may explode in heat of fire. Material emits nitrogen oxides when burned. Avoid carbon disulfide, silver perchlorate, imines, oxidizing materials. Stable. Hazardous polymerization may not occur.
-
使用用途
エチレンジアミンは、多くの化合物との反応性が高いため、新たな化学物質をつくる合成原料として使用されています。産業分野での例を挙げると、合成ワックス、除草剤、界面活性剤、乳化剤、湿潤剤、、腐食防止剤、洗剤、繊維表面処理などです。
医療分野の例では、抗ヒスタミン薬など化学的安定化剤として薬剤の合成、アレルギー性皮膚炎の診断を補助するためのアレルギー性上皮性パッチ検査など幅広い用途で使用されます。また、農薬関係では、殺菌剤、殺虫剤、除草剤などにも用いられます。
その他、キレート剤、コーティング剤、接着剤、イオン交換樹脂原料、ゴム薬品なども用途の1つです。
-
化学反応性
Reactivity with Water Gives off heat, but reaction is not hazardous; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
-
工業用途
EDA functions as a reactive intermediate in the synthesis of carbamate fungicides
and in the preparation of dyes, synthetic waxes, resins, insecticides and asphalt
wetting agents (Parmeggiani 1983). EDA is a solvent for casein, albumin, shellac,
and sulfur; an emulsifier; a stabilizer for rubber latex; an inhibitor in antifreeze
solutions; and a pharmaceutic aid (aminophylline injection stabilizer) (Windholz
1983). It is also an important ingredient in hair-settings, cold wave lotions, and
nail polish (Arena 1979).
-
接触アレルゲン
Ethylenediamine is used in numerous industrial processes
as a solvent for casein or albumin, as a stabilizer
in rubber latex, and as a textile lubricant. It can be
found in epoxy resin hardeners, cooling oils, fungicides,
and waxes. Contact dermatitis from ethylenediamine
is almost exclusively due to topical medicaments.
Occupational contact dermatitis in epoxy resin systems
is rather infrequent. Ethylenediamine can crossreact
with triethylenetetramine and diethylenetriamine.
Ethylenediamine was found to be responsible for sensitization
in pharmacists handling aminophylline suppositories,
in nurses preparing and administering
injectable theophylline, and in a laboratory technician
in the manufacture of aminophylline tablets
-
安全性プロファイル
A human poison by
inhalation. Experimental poison by
inhalation, intraperitoneal, subcutaneous,
and intravenous routes. Moderately toxic by
ingestion and skin contact, Experimental
reproductive effects. Corrosive. A severe
skin and eye irritant. An allergen and
sensitizer. Mutation data reported.
Flammable liquid when exposed to heat,
flame, or oxidizers. Can react violently with
acetic acid, acetic anhydride, acrolein, acrylic
acid, acrylonitrile, allyl chloride, CS2,
chlorosulfonic acid, epichlorohydrin,
ethylene chlorohydrin, HCl, mesityl oxide,
HNO3, oleum, AgClO4, H2SO4, Ppropiolactone, or vinyl acetate. To fight fwe,
use CO2, dry chemical, alcohol foam. When
heated to decomposition it emits toxic
fumes of NOx and NH3. See also MINES.
-
職業ばく露
Ethylenediamine is used as an intermediate; as a urine acidifier; as a solvent; an emulsifier for casein
and shellac solutions; a stabilizer in rubber late. A chemical
intermediate in the manufacture of dyes; corrosion inhibitors;
synthetic waxes; fungicides, resins, insecticides, asphalt wetting agents; and pharmaceuticals. Ethylenediamine is a degradation product of the agricultural fungicide Maneb.
-
製造方法
エチレンジアミンは、エチレンジクロライドとまたはアンモニア水との反応により生産されます。これらの原料を混合して、加圧下、110℃で加熱し反応させます。
ClCH2CH2Cl + 2NH3 / NH2H2CH2NH2 + 2HCl
反応により得られた生成物は、蒸留塔に送られます。そこで未反応のエチレンジクロライドは分離して反応槽へ戻されます。蒸留塔では40%苛性ソーダを振らせて、生成したアミン塩酸塩及び塩化アンモニアを中和し、遊離する過剰のアンモニアは反応塔に戻され再利用されます。
蒸留塔の底部から回収されるエチレンジアミンおよび食塩水を分離槽へ送り、分離槽で食塩水をエチレンジアミンから分離します。エチレンジアミンは精留塔で常圧、150~180℃で精製されます。残留分は沸点200℃以上のエチレントリアミン、およびポリアミンです。エチレンジアミンとトリアミン以上のポリアミンとの生成比率は2 : 1となります。
-
環境運命予測
Chemical/Physical. Absorbs carbon dioxide forming carbonates (Patnaik, 1992; Windholz et al.,
1983).
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent
concentration of 893 mg/L. The adsorbability of the carbon used was 21 mg/g carbon (Guisti et
al., 1974).
-
輸送方法
UN1604 Ethylenediamine, Hazard class: 8;
Labels: 8-Corrosive material, 3-Flammable liquid
-
不和合性
Vapor may form explosive mixture with
air. Ethylenediamine is a medium strong base. Violent reaction with strong acids; strong oxidizers; chlorinated organic
compounds; acetic acid; acetic anhydride; acrolein, acrylic
acid; acrylonitrile, allyl chloride; carbon disulfide; chlorosulfonic acid; epichlorohydrin, ethylene chlorohydrin,
oleum, methyl oxide; vinyl acetate. Also incompatible with
silver perchlorate, 3-propiolactone, mesityl oxide; ethylene
dichloride; organic anhydrides; isocyanates, acrylates,
substituted allyls; alkylene oxides; ketones, aldehydes,
alcohols, glycols, phenols, cresols, caprolactum solution.
Attacks aluminum, copper, lead, tin, zinc, and alloys; some
plastics, rubber, and coatings.
-
廃棄物の処理
Controlled incineration (oxides of nitrogen are removed from the effluent gas by scrubbers and/or thermal devices).