ChemicalBook > CAS DataBase List > Abemaciclib
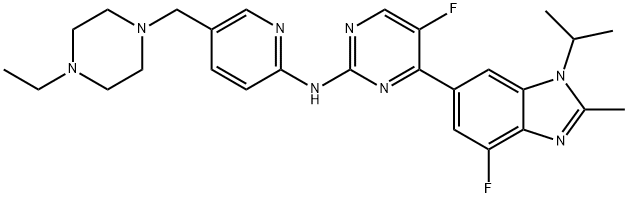
Abemaciclib
Abemaciclib
- CAS No.1231929-97-7
- Chemical Name:Abemaciclib
- CBNumber:CB72624906
- Molecular Formula:C27H32F2N8
- Formula Weight:506.59
- MOL File:1231929-97-7.mol
Abemaciclib Property
- Boiling point 689.3±65.0 °C(Predicted)
- Density 1.32±0.1 g/cm3(Predicted)
- storage temp. 4°C, protect from light
- solubility insoluble in H2O; ≥4.83 mg/mL in DMSO with gentle warming and ultrasonic; ≥6.34 mg/mL in EtOH with gentle warming
- form solid
- pka 7.69±0.10(Predicted)
- color Off-white to yellow
- InChIKey UZWDCWONPYILKI-UHFFFAOYSA-N
- SMILES C1(NC2=NC=C(CN3CCN(CC)CC3)C=C2)=NC=C(F)C(C2C=C3N(C(C)C)C(C)=NC3=C(F)C=2)=N1
- FDA UNII 60UAB198HK
- NCI Dictionary of Cancer Terms abemaciclib
- NCI Drug Dictionary abemaciclib
- ATC code L01EF03
Safety
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H373-H360-H410
- Precautionary statements P273-P391-P501-P260-P314-P501
Abemaciclib Chemical Properties,Usage,Production
-
Characteristics
Class: serine/threonine protein kinase
Treatment: Breast cancer
Oral bioavailability = 46%
Elimination half-life = 18 h
Protein binding = 95–98% - Uses Abemaciclib is a potent and selective inhibitor of cyclin-dependent kinases, CDK4 and CDK6, as a method to inhibit the proliferation of cancer cells.
-
Indications
Abemaciclib is used to treat breast cancer that is estrogen receptor-positive (ER-positive) and HER2-negative. It can be taken by both men and women. Patients may be offered abemaciclib if they have:
Early primary breast cancer that the treatment team thinks has a higher risk of coming back (recurrence). Abemaciclib may be provided as an adjuvant treatment, meaning a treatment given after initial treatment, such as surgery;
Breast cancer that has spread to the tissues and lymph nodes around the chest, neck, and under the breastbone (locally advanced breast cancer);
Breast cancer has spread to other parts of the body, such as the bones, lungs, liver, or brain (secondary breast cancer). - Biological Activity ly2835219 is an orally available cyclin-dependent kinase (cdk) inhibitor that targets the cdk4 (cyclin d1) and cdk6 (cyclin d3) cell cycle pathway, with potential antineoplastic activity. cdk4/6 dual inhibitor ly2835219 specifically inhibits cdk4 and 6, thereby inhibiting retinoblastoma (rb) protein phosphorylation in early g1. inhibition of rb phosphorylation prevents cdk-mediated g1-s phase transition, thereby arresting the cell cycle in the g1 phase, suppressing dna synthesis and inhibiting cancer cell growth. overexpression of the serine/threonine kinases cdk4/6, as seen in certain types of cancer, causes cell cycle deregulation.
- Biochem/physiol Actions LY2835219 (abemaciclib) was identified via compound and biochemical screening by scientists at Eli Lilly and Company Research Laboratories and selected for its biological activity and highly selective inhibition of the complexes CDK4/ cyclin D1 (IC50 =2 nmol/L) and CDK6/cyclin D1 (IC50 =10 nmol/L), with no activity against other CDK/cyclin complexes or cell-cycle-related kinases within the nanomolar ranges, except for inhibition of CDK9 at IC50 at least five times higher. The compound was shown to act as a competitive inhibitor of the ATP-binding domain of the CDK4 and CDK6 and to be 14 times more potent against CDK4 than against CDK6. In comparison to palbociclib and ribociclib, abemaciclib shows higher selectivity for the complex CDK4/cyclin D1, with IC50 values five times lower than those of the two other compounds[1].
- Mechanism of action Abemaciclib is a CDK4/6 inhibitor, and CDK4 and CDK6 proteins are closely related to stimulating cancer cell division and growth. Abemaciclib blocks the cell cycle and slows or stops cancer growth by binding to the ATP binding domain of CDK4 and CDK6, and it is 14 times more potent against CDK4 than against CDK6.
- Side effects Common side effects of abemaciclib include: gastrointestinal reactions (diarrhea, loss of appetite, nausea, vomiting, stomach pain and constipation); increased risk of infection (main symptoms include changes in body temperature, muscle aches, cough, headache, feeling cold and shivering, pain or burning when urinating); skin problems (rash, itchy and dry skin); bruises, bleeding gums or nosebleeds; difficulty breathing, paleness, taste changes, mouth pain, tiredness and weakness; hair loss. Serious side effects are fatal interstitial lung disease. In addition, abemaciclib can cause thrombotic coronary artery side effects when treating advanced breast cancer.
-
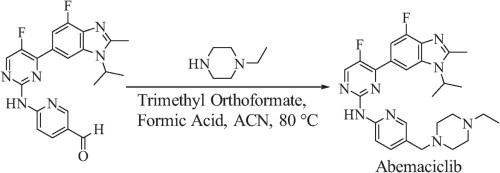
Synthesis
A concise total synthesis of CDK 4/6 inhibitor abemaciclib is described below. The synthesis uses a Suzuki coupling and a Hartwig–Buchwald amination to join three of the four subunits. The final step is a reductive amination utilizing Leuckart–Wallach conditions. Key to the Leuckart–Wallach reaction was the addition of trimethyl orthoformate to remove water formed during the reaction, allowing the reaction to complete[4].
- Enzyme inhibitor This oral cell cycle inhibitor (FWfree-base = 506.61 g/mol; FWmesylate-salt = 602.70 g/mol; CAS 1231930-82-7 (mesylate salt) ), also known as LY2835219 and N-[5-[ (4-ethyl-1-piperazinyl) methyl]-2-pyridinyl]-5- fluoro-4-[4-fluoro-2-methyl-1- (1-methylethyl) -1H-benzimidazol-6-yl]-2- pyrimidinamine, targets the cyclin-dependent kinase CDK4, or cyclin D1 (IC50 = 2 nM) and CDK6, or cyclin D3 (IC50 = 6 nM), inhibiting retinoblastoma (Rb) protein phosphorylation in early G1, thereby arresting the cell cycle in the G1, suppressing DNA synthesis, and inhibiting cancer cell growth. LY2835219 inhibits activation of AKT and ERK, but not mTOR.
-
References
[1] Silvia Paola Corona, Daniele Generali. “Abemaciclib: a CDK4/6 inhibitor for the treatment of HR+/HER2- advanced breast cancer.” Drug Design, Development and Therapy (2018): 321–330.
[2] “OS Trending Positive for Abemaciclib.” Cancer discovery 13 1 (2023): OF3.
[3] Erin R Scheidemann. “Resistance to abemaciclib is associated with increased metastatic potential and lysosomal protein deregulation in breast cancer cells.” Molecular Carcinogenesis (2024): 209–223.
[4] Michael O. Frederick, Douglas P. Kjell. “A synthesis of abemaciclib utilizing a Leuckart–Wallach reaction.” Tetrahedron Letters 56 7 (2015): Pages 949-951.
Abemaciclib Preparation Products And Raw materials
Raw materials
Preparation Products
Global(315)Suppliers
-
Supplier:
Beijing Hope Pharmaceutical Co., Ltd.
- Tel:+86-010-67886402<br/>+8613611125266
- Email:market@hopelife.cn
- Country:China
- ProdList:72
- Advantage:58
-
Supplier:
Arizest(Shanghai)Pharmatech Co., Ltd
- Tel:+86-021-60753300
- Email:3652837972@qq.com
- Country:China
- ProdList:220
- Advantage:58
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Cangzhou Kangrui Pharma Tech Co. Ltd.,
- Tel:+86-18632776803<br/>+86-13833998158
- Email:cangzhoukangrui@126.com
- Country:China
- ProdList:739
- Advantage:58
-
Supplier:
Guangzhou Tosun Pharmaceutical Ltd
- Tel:+86-020-61855200-902<br/>+8618124244216
- Email:info@upharm.cn
- Country:China
- ProdList:897
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
shandong perfect biotechnology co.ltd
- Tel:+86-53169958659<br/>+86-13153181156
- Email:sales@sdperfect.com
- Country:China
- ProdList:294
- Advantage:58
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
Related articles
1231929-97-7, AbemaciclibRelated Search:
- 1231929-97-7
- API
- Pharmaceutical
- Inhibitors
- 高纯化学试剂
- 高纯试剂
- 杂质对照品
- 抑制剂
- 抗肿瘤类
- 试剂
- 医药化工
- 药靶配体
- 医用原料
- API医药原料
- 医药原料药API
- 化学试剂
- 柏锦生物
- 普通产品
- 合成材料中间体
- 医药原料
- 玻玛西尼系列
- 小分子抑制剂,天然产物
- 小分子抑制剂
- 原料药
- 活性分子
- C27H32F2N8
- 阿贝西利杂质5
- 化合物ABEMACICLIB,10 MM DMSO 溶液
- 玻玛西林-[D8]/精确称量
- 阿贝西利游离碱
- 玻玛西尼/阿贝昔利
- ABEMACICLIB,CDK4/6 抑制剂
- 阿贝西利 ABEMACICLIB LY2835219
- 化合物ABEMACICLIB
- 阿贝西利游离
- N-[5-[(4-乙基-1-哌嗪基)甲基]-2-吡啶基]-5-氟-4-[4-氟-2-甲基-1-异丙基
- N-(5-((4-乙基哌嗪-1-基)甲基)吡啶-2-基)-5-氟-4-(4-氟-1-异丙基-2-甲基-1H-苯并[D]咪唑-6-基)嘧啶-2-胺
- 阿贝西尼
- 玻玛西尼
- 阿贝昔利布
- 阿贝西利
- N-[5-[(4-乙基-1-哌嗪基)甲基]-2-吡啶基]-5-氟-4-[4-氟-2-甲基-1-异丙基-1H-苯并咪唑-6-基]-2-嘧啶胺
- 1231929-97-7
- Abexili
- Abemaciclib, 10 mM in DMSO
- abecilee
- Abemaciclib Powder
- 5-(4-ethylpiperazin-1-ylmethyl)pyridin-2-yl)-(5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3H-benzimidazol-5-yl)pyrimidin-2-yl)amine
- inhibit,LY-2835219,LY 2835219,Abemaciclib,Inhibitor,Cyclin dependent kinase,CDK
- N-(5-((4-Ethylpiperazin-1-yl)methyl)pyridin-2-yl)-5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidin-2-amine
- N-[5-[(4-Ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl]-2-pyrimidinamine
- Abemaciclib (LY2835219)
- Abemaciclib D7
- Abemaciclib D10
- N-{5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}-5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-1,3-benzodiazol-5-yl)pyrimidin-2-amine
- LY2835219;LY-2835219;LY 2835219;ABEMACICLIB;CDK4/6 DUAL INHIBITOR;LY-2835219 FREE BASE
- CS-814
- Abemaciclib Mesylate (Verzenio)